Chemists started working with sulfonate esters back in the early 20th century, but the story of Trimethylsilyl Trifluoromethanesulphonate (often called TMSOTf) began gaining speed later. Research shifted gears during the mid-1900s when Lewis acid catalysts became tools to speed up reactions that would otherwise crawl. The commercial availability of trimethylsilyl-derived reagents pushed researchers past old boundaries. TMSOTf emerged as a strong silylating agent—its ability to activate oxygen atoms and stabilize transition states caught the attention of synthetic chemists looking to streamline their work. Papers from Japan and Europe began listing silyl triflates for applications beyond simple protection chemistry, which opened doors for TMSOTf to become a staple in academic and industrial labs.
Trimethylsilyl Trifluoromethanesulphonate’s clear, colorless liquid form tells little about how much punch it packs. Holding the formula CF3SO3Si(CH3)3, it acts with particular speed and strength among silylating reagents. Folks call it TMSOTf in the lab to save their breath. What TMSOTf brings to the table—versatility and reactivity—keeps it moving off chemical supplier shelves, with demand strongest in research labs pushing through complex syntheses, whether in pharmaceuticals or materials chemistry.
Trimethylsilyl Trifluoromethanesulphonate stands out with its boiling point around 100-102°C and a density a touch above water at roughly 1.2 g/cm3. It takes to most organic solvents—dichloromethane, ether, and acetonitrile—much better than to the open air, which quickly brings out its penchant for hydrolysis. Drop it in water, and the chemical ether vanishes, unleashing toxic triflic acid fumes. Its vapor carries a sour, biting odor that urges even old-timers to double-check their protective gear. Stability stays solid in cool, anhydrous conditions, but a careless cap or lab humidity can crack a bottle, turning an expensive investment into a lesson in caution.
Bottles of TMSOTf arrive with purity usually at or above 98%, marked clearly on supplier specsheets. Labels often carry the UN number for hazardous substances and note special handling needs: Corrosive, keep away from moisture, use in a fume hood. Manufacturers print batch numbers and recommended storage temperatures right on the label, which helps comply with global shipment regulations and trace any hiccups after purchase. Knowing your TMSOTf’s assay matters, as just a few tenths less purity can snowball into failed reactions—speaking from hours spent troubleshooting, it pays to demand a recent certificate of analysis before using a new batch.
Labs and factories make TMSOTf by reacting trimethylsilyl chloride with silver trifluoromethanesulfonate in a dry, inert atmosphere. The reaction looks clean on paper—one gives, one takes—but the process asks for careful temperature control and keen attention to exclude water. Silver salts come with cost and waste-disposal headaches, but the resulting TMSOTf stands tall for its purity and reactivity. Lab-scale work uses Schlenk techniques and gloveboxes to keep moisture out and to keep silver residues from spreading; on the factory floor, scale-up demands dedicated waste streams and careful tracking to turn out a reliable product fit for sensitive reactions.
TMSOTf owes much of its appeal to the way it transforms alcohols, amines, and other nucleophiles. It plucks the proton from an alcohol and swaps in a silyl group, creating silyl ethers, but at speeds and under milder conditions than many other agents. It activates carbonyls, boosts glycosylations, and turns basic building blocks into sturdy intermediates ready for more ambitious chemistry. Through careful selection of solvents and reaction partners, chemists find TMSOTf propels Friedel–Crafts alkylations, peptide couplings, and cyclizations that struggle with weaker catalysts. My own runs with TMSOTf in carbohydrate chemistry saved me days versus less reactive silylating reagents; the reagent never gave up its edge, even in the presence of acid-sensitive groups.
TMSOTf appears under several names depending on region and supplier. Beyond the abbreviations—TMSOTf, TMS-triflate—labels might show trimethylsilyl triflate or just trifluoromethanesulfonic acid trimethylsilyl ester. In catalogs from Europe to America, chemical codes make it easier to search and order, and every major supplier includes cross-references. This naming tangle trips up inexperienced buyers, but seasoned chemists recognize them all as roads leading to the same reactive liquid. Mistakes in ordering—or mixing up triflates—come at a cost, both to the wallet and the workflow.
Handling TMSOTf brings out the cautious side of every lab worker. Direct contact means burns and respiratory distress from the acid vapors. Spills corrode benchtops and make anyone nearby pay attention. Labs working with this reagent equip fume hoods, require chemical splash goggles, and keep calcium chloride tubes handy to intercept stray moisture. Training by mentors carries more weight than MSDS sheets because one slip with TMSOTf sticks in memory—my own mentor made sure gloves, goggles, and closed shoes lay between the bottle and bare skin. Spills need quick response and dedicated acid clean-up kits; even trace residues in the air prompt headaches or worse. Strict labeling and secure storage fight against casual accidents, but real safety grows from experience and constant vigilance.
TMSOTf finds more than a few jobs wherever protection, activation, or complex assembly happens. Medicinal chemists lean on it for preparing silyl-protected scaffolds when building drug candidates. Carbohydrate synthesis uses it to coax tough glycosylations along, producing oligosaccharides for vaccine and diagnostics development. Materials science teams apply it to craft specialty polymers or to modify surfaces, seeing the sharp, selective touch TMSOTf offers. Specialty cases turn up in peptide synthesis and natural product assembly, where stubborn target molecules yield only to the power of strong, selective silyl donors like TMSOTf. A single missed TMSOTf step can stall an entire drug project, highlighting how much modern synthesis relies on this versatile tool.
In the race to simplify synthetic routes and shrink environmental footprints, the spotlight stays on TMSOTf for both its advantages and faults. Teams look for ways to recycle silver byproducts or substitute cheaper salts in the preparation cycle, easing both cost and waste headaches. Collaborations between academic chemists and industrial developers hunt for new transformations unlocked by TMSOTf, often in greener solvents or under milder conditions. I’ve watched patents pile up around methods using TMSOTf in microgram drug syntheses or bulk commodity chemicals alike; its staying power comes from a balance of sheer reactivity and practicality. Ongoing work aims to tune selectivity, limit waste, and open new doors to bioactive molecules waiting for better bridges to their assembly.
TMSOTf brings the dual challenge of chemical burns and the formation of triflic acid, a strongly toxic, corrosive byproduct. Reports from exposure incidents stress the rapid onset of symptoms—skin blistering, eye damage, and respiratory problems. Toxicology studies associate direct contact with lasting injury, and the risk of inhaling vapors puts both acute and chronic toxicity on the table. Waste streams containing TMSOTf or its byproducts require neutralization and monitoring, since triflates bear persistence and mobility in soil and water. Regulations keep tightening around workplace exposure limits, and poison-control protocols demand information about silyl triflates and their effects. Labs develop local guidelines stricter than what’s written in guidelines—one mishap in handling shapes habits and rules for years to come.
The demand for TMSOTf sits snugly inside the drive for more efficient, sustainable synthesis. As new drugs and polymers grow more complex, the need for shortcuts and robust protection steps won’t slow down. Interest is building in more environmentally friendly derivatives and alternatives, but so far, nothing delivers the same speed or compatibility with sensitive substrates as TMSOTf. Digital chemistry modeling sheds light on new uses and better process controls. Ongoing development aims to reduce the expense and waste tied to its silver-based preparation, maybe by switching to catalytic systems or direct electrochemical methods. Each advance in green chemistry puts more pressure on the industry to adapt, but for now, TMSOTf holds its ground as a tool without equal for the frontline chemist. Experience keeps showing that, for all its hazards, careful work and creative research will keep unleashing new applications—and safer ways to handle them—in the shifting world of synthetic chemistry.
Trimethylsilyl trifluoromethanesulphonate carries a mouthful of a name, and anyone who’s worked with big molecules probably recognizes it by its catchier nickname, TMSOTf. This chemical doesn’t show up in news headlines, but walk into a research lab, and you’ll see scientists reach for it with confidence. What’s driving its popularity isn’t just tradition. It’s the reputation for being the go-to “silylating” agent and a strong promoter when tougher reactions stall out. My own days around the synthesis bench showed again and again: nothing else delivers quite like it in transforming the sluggish into the achievable.
Many chemists prize TMSOTf because it helps connect small building blocks into bigger, more complex molecules. In fact, whenever someone’s been talking about making new medicines, designing organic electronics, or building better plastics, you can bet TMSOTf has powered at least one synthetic step upstream. Its use as a silylating agent gives chemists a straightforward way to protect alcohol or amine groups during multi-step procedures, helping to keep those groups safe from harsh reactions down the line. A little shielding like this means more reliable results, lower waste, and better outcomes. Even with years of new technology flooding labs, TMSOTf’s dependable chemistry offers creative freedom for ambitious projects and complex molecule construction.
Chemists also grab TMSOTf when gentle options like trimethylsilyl chloride falter. TMSOTf acts with far more strength—it steps in when the job asks for more push. For example, the compound often sparks glycosylation reactions, which serve as the backbone for building carbohydrates or attaching sugars to drugs. That's hugely important in pharmaceuticals, especially in cancer drugs and vaccines where sugars switch on effectiveness. TMSOTf also makes short work of forming carbon-carbon bonds, a process essential for any company trying to scale up from lab prototypes to real-world products.
Having handled TMSOTf myself, I can’t ignore its dangerous side. This stuff stings eyes, burns skin, and really doesn’t play nicely with water or air—it’s volatile and, if handled the wrong way, can end a workday early for the wrong reasons. Proper gloves, careful work under a fume hood, and good storage habits really matter. Every chemist I know respects TMSOTf like they would respect a running chainsaw—useful, but you keep your guard up.
With all the positive outcomes, the frequent use of strong chemicals like TMSOTf means producers have to think about the bigger picture. If every lab pours used reagent down the drain, the impact shows up somewhere down the pipeline. Big manufacturers and university labs now look for greener methods, alternative reagents, and ways to recycle where possible. There’s active research on swapping in less hazardous chemicals that do much of the same job without the sharp risks.
Over the years, I’ve learned that picking the right tool—TMSOTf or otherwise—depends on weighing strengths against risks. In many cases, nothing else matches its punch and reliability. Good practice means not just knowing what a reagent can do, but always checking the safety data sheets, thinking about waste, and asking whether a safer option might work. Chemistry succeeds best when curiosity meets caution.
Trimethylsilyl trifluoromethanesulphonate, or TMSOTf to lab folks, sits on the shelf of many chemistry research labs. Known for its strong silylation power, it transforms the surfaces of molecules in a snap. Every chemist who’s opened one of these bottles knows the punchy aroma that follows—a sharp, almost shocking scent that means you better know what you’re doing.
TMSOTf reacts on contact with water, even with moist air. Leave the cap off for a moment or reach in with a wet spatula, and you trigger a mess. That reaction makes fumes you don't want in your face or your workspace. Lesson from many graduate students: always stay one step ahead of humidity. Always reseal containers right away and use sealed vials for any splitting of quantity.
Cold storage helps, but fridges often carry their own risks of condensation and accidental spillages. I’ve seen bottles double-bagged inside small, acid-resistant boxes. A dry, cool cabinet with low humidity and stable temperature usually works best. Keep it away from basic reagents and water sources; don’t cram it in with open bottles of sodium hydroxide or glassware that's been washed and not fully dried.
Glass vials with airtight PTFE-lined caps help avoid leaks and keep the reagent from decomposing. I ran into troubles once using a plastic cap—turned brittle, warped, and pretty soon the bottle wouldn’t close right. Swap out caps if you spot any cracks. Always put a label with clear dating, your initials, and hazard warnings visible from every angle.
Accidental spills end up more common than you’d hope. Small drops smoke up in the presence of any moisture. Always keep absorbent pads right next to where you open TMSOTf and have neutralizing agents nearby. You don’t want to fumble around when it’s already making a scene on your benchtop. If you spill it, neutralize with sodium bicarbonate—slowly and in the fume hood, since it can fizz up quickly.
Splash goggles, gloves, and a buttoned-up lab coat: those are not up for debate. TMSOTf eats through ordinary nitrile gloves faster than a lot of people expect, so double-gloving or using thicker lab gloves can lower risk. Keep only what you need for your experiment on the bench, and return the bulk bottle to storage as soon as you’re done. Never use it outside a fume hood. Direct inhalation, even for a moment, brings a burning, choking feel no one forgets.
Dispose of any leftovers in well-labeled halogenated waste containers. I always recommend double-checking your institution’s disposal guide, since mistakes can haunt you or the next shift. The reaction byproducts and trace fumes can corrode metal, so keep your workspace clean and check all clamps, racks, or stir bars regularly for any sign of trouble.
It’s one thing to skirt the rules and hope for smooth sailing. Over time, shortcutting proper storage and handling leads to bigger headaches—a ruined experiment, a contaminated hood, or personal injury. Team up with colleagues to set up visible checklists near storage areas. That visible reminder beats a forgotten warning any day.
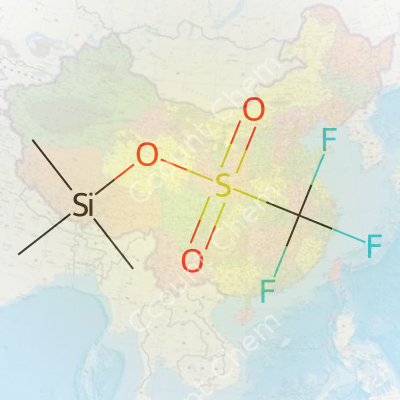
Trimethylsilyl trifluoromethanesulphonate, often called TMSOTf in laboratories, carries the chemical formula C4H9F3O3SSi. This compound shows up at the bench whenever chemists need to push forward with an efficient silylation. It's got tough-sounding chemistry, but this reagent works as a silent workhorse for a wide swath of synthetic transformations.
The molecular structure comes together with four carbon atoms, nine hydrogens, three fluorines, three oxygens, a single sulfur, and one silicon atom. That lands TMSOTf with a calculated molecular weight of 236.26 grams per mole. The presence of three fluorines and a sulfonate group gives the molecule a "kick," making it reactive in ways that other silylating agents can’t always match.
Years spent in research labs teach you respect for these distinct features. The trimethylsilyl moiety (Si(CH3)3) quickly attaches to oxygen or nitrogen atoms, helping protect sensitive groups in a reaction. Protection with TMSOTf can mean the difference between a successful synthesis route and a failed one. Quick reactivity, low side products, strong leaving group capability—all tie right back to the unique formula and weight.
TMSOTf’s strong silylating power helps streamline synthesis, especially in pharmaceuticals and speciality chemicals. A chemist looks at the formula and sees more than numbers: that trifluoromethanesulphonate group activates the silicon, pushing silylation on sluggish partners and stubborn functional groups. Conventional silyl chlorides often require longer times and extra coaxing. The efficiency here means a shorter pathway to new drug scaffolds, agrochemicals, and advanced materials.
Yet such reactivity heightens risks. Volatility and moisture sensitivity require respect. Any water in the system burns through your valuable reagent, forming unwanted byproducts and short-circuiting the desired reaction. My own work with TMSOTf reminds me to check for dry glassware twice, not just once. You save time and resources that way, and you ensure the end product meets quality standards. TMSOTf’s chemical makeup forces careful lab technique, which raises the bar for safety and reproducibility in chemical research.
Storage and handling demand close attention. Keep TMSOTf away from sources of water—it hydrolyzes fast. Glass vials, septa, and syringes all deserve a thorough oven-drying before touching this compound. Labs often store TMSOTf under inert gas like nitrogen or argon. Any slip means loss of expensive material and risks to health or the environment.
Personal protective equipment isn’t just a suggestion. TMSOTf vapors can irritate the respiratory system and eyes. Good airflow in fume hoods, gloves, goggles, and swift cleanup of spills support safe handling. Teams who train with this level of care not only protect themselves, but also deliver more reliable, consistent research results.
TMSOTf synthesis and use relate to the broader issue of responsible chemical development. Minimize waste by scaling reactions carefully and recycling solvents wherever possible. Labs around the world increasingly favor greener processes. Planning begins with understanding your materials—knowing the formula and precise mass guides calculations, ordering, and waste reduction. That’s one simple but significant step in making sophisticated science a little more environmentally friendly.
Familiarity with TMSOTf’s chemical and physical details informs decisions from the bench to the boardroom. Getting the hands-on chemistry right clears a path for better, safer, and smarter synthesis today and tomorrow.
Trimethylsilyl trifluoromethanesulphonate, often shortened to TMSOTf, gets most of its attention in organic synthesis. This stuff acts as a strong silylation and alkylation agent. Step into any research lab, and you’ll likely find chemists using it to build or modify delicate molecules. The science is impressive, but the real story lies in how this clear, colorless liquid holds both promise and potential for harm. Having worked with it, I can tell you the safety sheet doesn’t exaggerate TMSOTf’s bite.
If TMSOTf touches skin, irritation follows, sometimes with burning and redness that goes beyond a simple rash. Eyes fare far worse—splashes can lead to permanent damage. I learned early to double up on gloves and never skimp on the goggles. Researchers occasionally rush or get careless. That’s when exposures occur. Chemical-resistant gloves and face shields stop being overkill after seeing a spill eat through nitrile or create a white haze in someone’s safety glasses.
Anybody who’s cracked open a fresh bottle of TMSOTf never forgets the smell—sharp and chemical, almost aggressive. Vapor exposure leads to coughing fits, irritation, and sometimes headaches. Fume hoods aren’t a suggestion here; they’re essential. A slight spill can fill a room with vapors. Laboratories with poor ventilation invite coughs and mistakes. Occupational Safety and Health Administration (OSHA) regulations are in place for good reason, as repeated inhalation can cause long-term respiratory issues.
TMSOTf does not play well with water. In humid air or contact with even a droplet, it produces triflic acid—a superacid known for corroding glass and burning through skin. During my grad school days, an impatient colleague added TMSOTf to a reaction mixture with trace water. The fizzing and heat proved how fast the situation could turn ugly. Quick thinking and a neutralizing agent prevented burns, but the memory stuck with us all. Equipment must stay bone dry, and anyone handling this stuff gets a strict lecture on proper storage and disposal.
Although TMSOTf itself doesn’t catch fire easily, combining it with flammable solvents adds another layer of risk. Once a reaction runs amok, the combination can ignite, especially if acids or bases mix in. Most labs keep solvents handy, so open flames or spark sources nearby increase the risk. Fire extinguishers and spill kits should never be pushed to the back corner or neglected.
Getting rid of unused TMSOTf isn’t as simple as pouring it down the drain. The compound reacts with water in waste pipes, releasing toxic fumes and acids that corrode infrastructure. Environmental Protection Agency (EPA) rules require neutralization and collection in sealed, vented containers until professionals can treat or dispose of it safely. Labs that ignore this leave both people and the neighborhood water supply at risk.
Minimizing hazards starts with proper training. Clear signage, detailed protocols, and hands-on mentorship keep young researchers from making costly mistakes. Personal protective equipment stands as non-negotiable, not a backup. Well-maintained fume hoods and emergency showers create an extra layer of security. Institutional commitment to regular safety audits catches small lapses before they lead to accidents. Safety may feel tedious or slow research, but with compounds like TMSOTf, it keeps everyone healthy and labs running.
Trimethylsilyl trifluoromethanesulphonate, often called TMSOTf in labs, plays a critical role in organic chemistry. Its reactivity brings a real punch to many syntheses, but not many talk about what happens after pouring it out of a flask. Anyone who’s worked around this stuff knows: this isn’t a “pour down the drain and call it a day” situation. Getting careless with disposal can turn a good experiment into a safety emergency.
This chemical reacts aggressively with water. You add a drop to a beaker with a hint of moisture, and white, noxious vapors leap out. Those vapors include triflic acid, one of the strongest acids you’ll ever meet outside of a research journal. That makes direct flushing or tossing into a regular waste stream dangerous for anyone nearby. Inhaling triflic acid mist or letting it contact skin ranks high on the list of lab mishaps—you just don’t want to risk it.
I remember my first time handling the residue from a TMSOTf reaction. The senior chemist didn’t just wave me over to the sink. Instead, he pointed to a sealed drum labeled "halogenated organics only." He had scars on his hands, and stories about what happens when someone cuts corners with reactive waste. It stuck with me. Responsible disposal starts right at the bench with how you neutralize and contain the leftover material—before it ever leaves your workspace.
Labs storing small amounts can collect all TMSOTf waste in a glass bottle, never plastic, since the chemical breaks down certain plastics. Always segregate TMSOTf from aqueous or basic waste streams. Combining wastes triggers exothermic reactions, and sometimes spontaneous fires. Take it from someone who’s seen bench-tops smoulder and warranties on fume hoods voided in minutes.
Trust isn’t about hoping for the best. The CDC and EPA highlight that anything this reactive falls under the hazardous waste regulations laid out in the Resource Conservation and Recovery Act (RCRA). That means you collect TMSOTf waste, clearly label it, and call for a licensed chemical waste contractor. They know how to neutralize or incinerate this stuff with proper containment.
Neutralization with water in a blast-shielded hood is possible for very small amounts, but triflic acid forms almost instantly. That side product eats through metal, burns through organic matter, and destroys pretty much any unprotected surface. Staff must suit up with heavy gloves, splash goggles, face shields, and work under a well-ventilated hood. Even then, labs usually stick with professional waste pickup for anything over a few milliliters.
Look for a written chemical hygiene plan at every lab using TMSOTf. If you walk into a facility and nobody can show you a spill kit or eyewash station, you’re in the wrong place. All staff need regular training, not just written handouts. Emergency protocols should sit right next to the spill control gear.
Many university departments partner with licensed disposal services to audit their chemical workflow every year. They adjust waste handling procedures as regulations shift, or as student safety concerns come up. If your lab’s list of contact numbers doesn’t include local hazardous waste teams, push for an update.
It takes more than technical know-how to stop these chemicals from ending up in waterways or landfills. Hazmat teams trace back environmental damage to improper lab disposal every year. If your work generates reactive chemical waste, you own the duty to prevent that outcome—no excuses. A good chemist never leaves waste for someone else to clean up.
So, if you’re handling trimethylsilyl trifluoromethanesulphonate, you owe it to your colleagues and your community to know how to dispose of it right. It may slow your project down, but that extra care means fewer emergencies, safer labs, and a cleaner environment beyond the research doors.
| Names | |
| Preferred IUPAC name | trimethyl(trifluoromethanesulfonoxy)silane |
| Other names |
TMSOTf Trimethylsilyl triflate Trimethylsilyl trifluoromethanesulfonate Trimethylsilyltrifluoromethanesulfonate |
| Pronunciation | /traɪˌmɛθ.ɪlˌsaɪl.i traɪˌflɔːr.oʊˌmɛˈθeɪnˌsʌlˈfəʊ.neɪt/ |
| Identifiers | |
| CAS Number | 27607-77-8 |
| 3D model (JSmol) | `3D model (JSmol) string` for **Trimethylsilyl Trifluoromethanesulfonate** (CAS 27607-77-8): ``` C[Si](C)(C)OS(=O)(=O)C(F)(F)F ``` This is the **SMILES** string that can be used to generate the JSmol 3D model. |
| Beilstein Reference | 87355 |
| ChEBI | CHEBI:87777 |
| ChEMBL | CHEMBL155488 |
| ChemSpider | 18700 |
| DrugBank | DB11272 |
| ECHA InfoCard | 100.063.858 |
| EC Number | 214-589-7 |
| Gmelin Reference | 81187 |
| KEGG | C14146 |
| MeSH | D013984 |
| PubChem CID | 66417 |
| RTECS number | XZ1925000 |
| UNII | FT9SWT2JWH |
| UN number | UN3265 |
| Properties | |
| Chemical formula | C4H9F3O3SSi |
| Molar mass | 282.32 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.332 g/mL at 25 °C |
| Solubility in water | Hydrolyses |
| log P | 0.1 |
| Vapor pressure | 7 mmHg (20 °C) |
| Acidity (pKa) | -12.2 |
| Basicity (pKb) | Basicity (pKb): -23 |
| Magnetic susceptibility (χ) | -62.5 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.338 |
| Viscosity | 2.11 cP (20°C) |
| Dipole moment | 3.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 348.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1106.6 kJ mol⁻¹ |
| Hazards | |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H225, H314, H302, H332 |
| Precautionary statements | P210, P233, P260, P264, P271, P280, P304+P340, P305+P351+P338, P312, P330, P337+P313, P403+P233, P501 |
| NFPA 704 (fire diamond) | 1-3-0-W |
| Flash point | 37 °C |
| Autoignition temperature | 250°C |
| Explosive limits | Lower: 1.0% Upper: 7.6% |
| Lethal dose or concentration | LD50 Oral - rat - 933 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 1700 mg/kg |
| NIOSH | TTQ40270 |
| PEL (Permissible) | PEL (Permissible exposure limit) for Trimethylsilyl Trifluoromethanesulphonate is not established. |
| Related compounds | |
| Related compounds |
Methanesulfonic anhydride Trifluoromethanesulfonic anhydride Trimethylsilyl chloride Trimethylsilyl iodide |