Trifluoromethanesulphonic anhydride, often referred to by many as triflic anhydride, started to catch attention in synthetic chemistry circles in the mid-twentieth century. Pushback against harsh chemical environments and limited scope of sulphonation routes led researchers to look for more reactive, cleaner sulfonating agents. Early patents trace to the drive for reagents that could introduce trifluoromethanesulfonyl (triflyl) groups smoothly, leaving behind only volatile and unreactive byproducts. At that time, chemists leaned on unpredictable mixtures like sulfuric/trifluoromethanesulfonic acid blends before the cleaner, isolated anhydride compound took over as the leading reagent, especially in peptide synthesis and complex small molecule construction. The cost of fluorine chemistry once priced out small labs, but expanded industrial fluorination capacity in the 1980s and 1990s opened doors for wider use.
Ask anyone who has worked in a chemical synthesis lab about this compound, and the phrase “triflic anhydride” brings out nods of respect—and not a little caution. Here is a liquid that pours almost water-clear, but packs a punch, making it a go-to for activating otherwise stubborn substrates. Unlike triflic acid, which arrived as an ultra-strong acid in the mid-1900s, its anhydride brings reactivity without the water—no dilute mess, only pure, fast results. Chemists use this tool when they can’t afford a trace of water, and need to transfer the triflyl group directly. Suppliers label the bottle as a colorless to pale yellow liquid, sharply reactive, with a fluorinated fume that tells you not to breathe near it.
In practical lab terms, triflic anhydride flows as a clear, oily liquid, boiling at roughly 81 °C, with a freezing point near -80 °C that stores easily over standard conditions but never near open water or amines. Weighing in heavy, thanks to the three fluorine atoms, its vapor creeps quickly. Its smell smacks sharp and stinging, hinting at the strength packed in its molecular structure. As a symmetrical anhydride, it reacts instantly with water, and generates not only the extremely strong triflic acid but also feverish heat. Faced with most nucleophiles, it transforms weak bases into strong triflate leaving groups, a change that speeds up many reactions by orders of magnitude.
Commercially, triflic anhydride usually comes in sealed glass bottles, with labels marked “Corrosive,” “Dangerous upon contact with water,” and the vital UN numbers for transport. Purity often sits above 99%, and because of both reactivity and volatility, suppliers ship it with drying agents or under inert gas pads. Lab users check for clarity, absence of discoloration, and confirm moisture content is below 0.05%, since any water ruins selectivity. Sigma-Aldrich, Tokyo Chemical Industry, and Alfa Aesar set the bar on specification sheets, pointing out trace metal contents, which matter for sensitive electronic or pharmaceutical uses.
Commercial plants put together triflic anhydride from the dehydration of trifluoromethanesulfonic acid. In a lab, strict dryness dominates—the whole process closes out air and water by using sealed glass reactors under inert gas. Common dehydration agents include phosphorus pentoxide, sometimes acetic anhydride, or special reagents tailored for production scale. Each batch requires careful distillation under vacuum, since heat triggers decomposition that fills hoods with white fumes and can ruin a week’s work. Contaminants from incomplete dehydration or metallic catalysts cost good product, so quality control at this stage directly hits the bottom line, especially for electronics or pharma use where purity translates to reliability.
In synthetic labs, triflic anhydride plays catalyst, activator, and transformer. It finds itself at the heart of triflation, converting hydroxyl, amine, and carboxyl groups into their much more reactive triflate forms. Strong base, like pyridine or 2,6-lutidine, gets added to mop up byproducts and keep reactions clean. In Friedel-Crafts acylation, switching to triflic anhydride often fixes sluggish reactions that would otherwise fail with weaker acids or sulfonates. But its versatility extends beyond: it enables activation of unreactive carbonyls, triggers cyclizations, and unlocks peptide coupling steps that stall with other anhydrides. Organometallic chemists count on its gentler touch for generating organotriflate intermediates with sensitive functional groups intact.
Over the years, the official nomenclature ran long—trifluoromethanesulphonic anhydride—so chemists trimmed it to “triflic anhydride” or “triflyl anhydride”. Product catalogs mark it under CAS number 358-23-6, and most research papers stick with “Tf2O”. Some older European texts call it “perfluoromethanesulfonic anhydride,” but the chemistry community mostly dropped that in favor of the triflic name for clarity.
Triflic anhydride’s hazards go beyond splash-and-burn. Even at room temperature, direct skin or eye contact means a trip to the emergency room, and vapors damage lungs without proper ventilation. Fume hoods, neoprene or nitrile gloves, and well-fitted goggles rule in handling routines. Spills get neutralized not with water—never with water—but with dry sodium carbonate. Staff receive training in chemical hygiene, and storage rules demand separation from water, bases, and anhydrous reagents. Industrial settings lock this compound in explosion-proof cabinets, with fire suppression and emergency eye wash at arm’s reach. Disposal requires neutralization and collection under local environmental regulation, as both acidity and fluorination pose risks for downstream water systems. Companies emphasize written protocols, and government agencies like OSHA in the US and REACH in Europe set exposure limits, document risk, and enforce proper labeling.
Walk through a synthetic organic lab working on new pharmaceuticals, and triflic anhydride shows up all over the bench. Medicinal chemists love its ability to convert building blocks into triflates, laying a foundation for further reactions. In peptide chemistry, Tf2O helps make tough connections without decomposing other sensitive regions. Beyond drugs, in the electronics sector, it allows clean sulfonation steps on polymers for high-purity, moisture-resistant components. The push for green and efficient transformations in the chemical industry also benefits from its reactivity, reducing side products and minimizing purification steps. Suppliers report growth in demand from manufacturers of specialty polymers, OLED precursor producers, and custom reagents for fine chemicals. In academic research, it fattens spectra and fills journals with new ways to functionalize previously “unreactive” molecules.
Academic and industrial R&D teams keep hunting for even faster, cleaner, and safer variants. Articles in journals like JACS and Angewandte Chemie show teams tying triflic anhydride into automated chemistry and flow-reaction manifolds. Labs push for catalytic processes that use smaller, safer doses, trying to minimize waste and avoid costly downstream neutralization. In recent years, creativity shifts toward merging triflic anhydride steps with photochemistry or biocatalysis—unlikely partners ten years ago. Major chemical companies like Merck and BASF pour funding into systems for recycling byproducts and designing safer triflyl agents that keep potency but reduce personal risk. On the education front, undergraduate labs teach remote reaction monitoring via webcam as a way to introduce this reagent without direct student exposure.
Toxicity data for triflic anhydride tells a cautionary tale. Short-term exposure, even at low concentrations, leads to serious respiratory irritation, chemical burns, and, at high doses, systemic toxicity. Animal tests indicate possible organ toxicity and delayed healing due to persistent sulfonic acid residues, and studies on chronic exposure show corrosion of tissue and elevated risk of lung damage among operators. Industry pushes for protective equipment and medical surveillance where exposure risk runs high. Despite the risks, few long-term mutagenicity or carcinogenicity studies exist, but experts urge better documentation due to increasing industrial scale and broader use. Regulators and chemical manufacturers fund research to close the safety data gap, especially to set global guidelines and help small labs protect workers without the resources of larger corporations.
Every year, new patents list potential greener analogs and milder triflylating strategies based in part on the performance of trifluoromethanesulphonic anhydride. The challenge lies in holding onto its efficiency and power while lowering both personal and environmental risk. Research teams explore immobilized reagents that lock away fumes and acids, re-engineer classic batch processes into continuous flow to shrink waste, and search for catalysts that extend the triflic method to water-sensitive drugs materials. Digital labs and AI-led screening speed up safety data collection and process optimization, so twenty years from now, the hope is for a toolkit that keeps the transformative reactivity of Tf2O, applies it safely at all scales, and fits into a future where sustainability and worker wellbeing take center stage. From fine chemical synthesis to next-gen electronics, the chemical world will keep building on the lessons and breakthroughs born from this feisty, high-impact reagent.
Trifluoromethanesulphonic anhydride doesn’t exactly catch the public eye, but folks working in chemistry or drug development know the value packed inside a small flask of this stuff. In the chemistry world, people often call it “triflic anhydride.” It’s strong and reactive, known for its knack in making tough chemical tasks a bit easier. Back in college, I helped out on a project that relied on this chemical to get complicated molecules to shape-up into something useful, and I still remember how one misjudged drop threw off a whole week’s work.
Why do chemists keep turning back to triflic anhydride? Reliability, speed, and power. It activates certain chemical bonds that normally refuse to budge, especially in aromatic rings. Take the process of installing “triflate” groups onto molecules—this one step can set off a chain of possibilities for building medicines, new materials, or crop protection products. Some drugs wouldn’t exist in today’s world without the punch this reagent brings to the table. For example, triflic anhydride plays a key role in preparing compounds for antiviral drugs or painkillers.
Let’s break it down. Triflic anhydride handles jobs that regular lab acids can’t. Its proven track record in chemical synthesis comes from being one of the most aggressive agents for activating alcohols, converting them into triflates, which are then used to build complex molecules through cross-coupling reactions. If you root around in patents or scientific papers, traces of triflic anhydride show up everywhere, from making advanced plastics to fine-tuning dyes or liquid crystal materials. These are not fringe uses—they keep entire research labs and production floors busy.
Safety needs a mention too. Triflic anhydride works with muscle, but a chemist must treat it with respect: protective gloves, good ventilation, and careful handling. A spill or whiff can go sideways quickly. Its harsh nature is what makes it so good at its job, but also earns it a spot on hazard lists. Everyone involved in its use, from shipping to disposal, carries real responsibility. I learned this firsthand after seeing a coworker mishandle a bottle—an experience that drove home the strict procedures built around chemicals like this.
Every powerful tool needs a watchful eye. Triflic anhydride lands on regulatory radar in many regions. Labs arrange special storage, track quantities, and manage waste through licensed disposal services. People are searching for greener, less hazardous alternatives, but nothing else in the toolkit matches its punch in some key reactions. Companies investing in better fume hoods and fast emergency showers do so not as a luxury, but as an answer to the real dangers that come with the chemical.
Reducing risk and pollution fall on everyone working with triflic anhydride. Universities and pharmaceutical companies can support research into substitutes or safer workarounds. Open sharing of safety data and reaction tricks saves time and, sometimes, lives. My own experience in academic labs showed me how much difference clear guidelines and peer oversight can make for newcomers learning the ropes.
Trifluoromethanesulphonic anhydride ranks as a heavyweight in chemical synthesis circles. Its reliability has built trust over decades, but comes with non-negotiable safety and environmental responsibilities. People in and outside labs have a role in developing processes that are as safe and efficient as possible, whether through better training, equipment, or by supporting the hunt for next-generation reagents that carry less baggage. For now, this chemical stays essential in shaping modern medicines and materials, with all eyes open to both its promise and its risks.
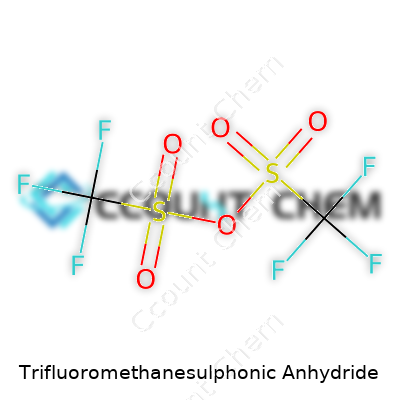
Trifluoromethanesulphonic anhydride draws attention from synthetic chemists because it offers power and precision for activating chemicals in the lab. The formula wraps up in simple terms: (CF3SO2)2O. Looking at its skeletal structure, the molecule connects two trifluoromethanesulfonyl groups through an oxygen atom. Chemists visualize this as two CF3SO2- units flanking an oxygen atom—imparting it with symmetry and steric heft. Each trifluoromethanesulfonyl group hooks up via a sulfur atom, each sulfur doubling up with two oxygen atoms and a single oxygen bridge. The fluoroalkyl arms (CF3) draw in that high electronegativity, turning the molecule into a one-two punch of reactivity and selectivity.
Working with this reagent, or just discussing its chemistry, shifts conversations. Chemists use it much the way a chef depends on sharp knives. Every good organic or medicinal chemist learns to respect what "Tf2O" can do. With the size of the trifluoromethyl group and the strong leaving power granted by the sulfonyl group, the molecule brings an intensity for activating alcohols, setting up sulfonates, and forging reactive intermediates with almost framework-like reliability. Making triflates (trifluoromethanesulfonates) out of wobbly alcohols ramps up transformation potential in cross-coupling or substitution reactions.
It's one thing to understand the structure. The true appeal comes from what Trifluoromethanesulphonic anhydride enables in the lab. As someone who’s navigated the tricky waters of building compounds with unusual shapes or functions, using this reagent cuts through barriers, creating pathways where none seemed possible. It transmutes less reactive molecules into ideal substrates, smoothing the way for tricky Suzuki or Stille couplings.
In the lab, Trifluoromethanesulphonic anhydride brings an aggressive edge. Sharply corrosive, it demands respect and triggers careful planning well before reaching for a pipette. Any hint of moisture ruins its potency, so chemists store it under dry inert gas. Full personal protective equipment and robust fume hood use are non-negotiable steps. Having once underestimated the fumes from a sulfonyl chloride, I learned firsthand the fallout: burned eyes and wasted material. The need for rigorous handling isn’t academic—it's rooted in keeping both chemist and chemistry intact.
Much as the molecule revolutionizes chemical synthesis, producers weigh environmental impacts seriously. The fluorine atoms embedded in its structure carry persistence, making waste streams a real challenge if not tightly regulated. Research groups and corporations look towards greener synthesis, aiming to harness its power but slash emissions and accidental releases. Tighter protocols, smaller scale-up runs, and better chemical capture schemes speak to a growing awareness that efficiency in the flask must match responsibility in waste management.
While Trifluoromethanesulphonic anhydride stays enshrined as a top-tier activating agent, chemists continue searching for alternatives that balance power with sustainability. Investing in research for milder, less persistent reagents could yield chemicals just as reactive but kinder to the environment. As demand for complex, high-functionality molecules grows in fields like pharmaceuticals, the tools borrowed from traditional playbooks need updating to match both technical and ethical expectations.
Trifluoromethanesulphonic Anhydride packs a punch in labs, thanks to its strong reactivity. If this stuff splashes, fumes, or even spills, it doesn’t cut corners—it causes real burns and chokes when mishandled. It reacts quickly with water, releases toxic gases, and poses threats to anyone who isn’t playing it safe. The real trouble starts with exposure. Direct contact with skin, eyes, or lungs sets off reactions that nobody forgets. That’s why anyone around it should know what they’re dealing with.
Every lab that uses triflic anhydride has stories about what happens without proper storage. Corrosion, leaky containers, and contamination aren’t rare—they’re the norm for those who get casual. I once saw a bottle start eating through the shelving after a poorly sealed cap. What stops these headaches is a smart system. Tough containers—usually glass or tough plastic—and a clear rule: keep water far away. Dedicated cabinets, labeled for toxic and reactive chemicals, work best. If a storage spot has high humidity or gets used for food or drink, it doesn’t belong there. Even one mistake brings problems that last for weeks.
The lessons stick hardest when you see a spill up close. Fumes can get out fast, react with air or water, and leave everyone scattered. Full-face shields, goggles, thick gloves, and a chemical apron form the basic getup—skip one, and you’re gambling health for speed. There’s no such thing as too cautious. Preparing spill kits with neutralizing agents, dry sand, and absorbent materials gives a backup plan. No one wants to grab paper towels in a real emergency. Eye wash stations and safety showers near the workspace should never get blocked by carts or boxes. Good ventilation isn’t optional; fume hoods make a real difference. Trust me, regular air circulation won’t cut it—you need the right setup to avoid breathing in dangerous fumes.
Some folks try to ignore cleanup rules, thinking a rinse is enough. I once watched a lab tech ditch some triflic anhydride waste down the sink. The drains corroded, the smell lingered for days, and the repair bill landed hard. Neutralize leftovers with the right agent—often an ice-cold basic solution, carefully added, not dumped. Mark containers for hazardous waste and call in licensed disposal services. City water systems were never built to handle this type of chemical, and the fallout hits both workers and the environment. Reporting accidental releases isn’t just about paperwork—it keeps everyone in the loop, limits damage, and makes sure help shows up if needed.
Every new person in a lab should hear real-life stories about mistakes before they make their own. This isn’t just theory—sharing what happens when someone forgets goggles or leaves a cap loose makes a real difference. Labs thrive on teamwork. No one should handle this chemical without support and double-checks. Written procedures mean nothing if they sit in a drawer. Get everyone comfortable with asking questions, running emergency drills, and speaking up about what feels risky. Chemicals like triflic anhydride won’t forgive shortcuts, so building a culture of steady, clear-eyed respect is the only way forward.
Anyone who’s worked with strong sulfonating agents knows mishandling Trifluoromethanesulphonic Anhydride, often called triflic anhydride, can kick up considerable trouble. This is a colorless, oily liquid that goes beyond the bite of most everyday acids. It earns a reputation as an aggressive sulfonylating and activating agent.
Direct contact with this chemical often leads to severe burns. Vapor exposure easily irritates eyes or the respiratory system, and a splash to the skin brings pain quickly. Inhalation triggers coughing and choking, even at low concentrations, since the fumes are sharp and reactive. Spills on a benchtop tend to smoke or fume in the presence of water, which can quickly spiral from a minor accident into an all-out containment issue.
Corrosivity isn’t limited to flesh. Triflic anhydride chews up glassware not designed for corrosives and will react with water, alcohols, and even trace moisture in a sealed bottle. If you leave the cap loose, it doesn’t take long for vapors to corrode metal fixtures or equipment around the fume hood.
Every bottle carries acute toxicity and, based on studies, long-term effects remain unclear. Regulatory resources highlight the chemical’s dangerous reactivity with bases, nucleophiles, and flammable solvents. Ignoring safety steps can leave behind chronic respiratory issues or deep burns that take weeks to heal.
Triflic anhydride’s volatility also raises concern about environmental release. Drain disposal can spark fires and vapor clouds, while spills on the floor threaten anyone in the area. I remember seeing a colleague’s glove dissolve seconds after a small splash—a reminder that nitrile alone doesn’t cut it here.
Working with triflic anhydride calls for discipline every time, not just during big reactions. Full-length chemical-resistant gloves—think heavy-duty Viton or butyl—outperform ordinary lab gloves. Face shield and goggles matter more than with most reagents, since even vapor off the bench can sting eyes or nose. Lab coats only go so far; chemical-resistant aprons step in to protect against unexpected sprays. Never skip the fume hood, even for short transfers.
Before and after use, make sure containers get checked for moisture. Drying tubes packed with calcium chloride or phosphorus pentoxide keep the contents stable between uses. If a bottle feels sticky or shows discoloration near the seal, it’s time to replace it. If you spill even a drop, specialized spill kits for acids—not just basic absorbents—get things under control fast. Rinse all disposal glassware with copious amounts of cold water before anything gets decomposed or neutralized.
Training plays a major role. Refresher courses force open conversations about chemical hazards. Signs and protocol sheets inside the lab stay current. Share stories about shortcuts gone wrong; these firsthand examples help new team members recognize the risks firsthand rather than learning the hard way.
In my own experience, slow, methodical work and calling for a spotter in tricky transfers beats racing the clock. Pinning down the safest sequence and prepping every tool ahead of time reduces stress and slip-ups. At the end of the day, respecting chemicals like triflic anhydride doesn’t slow research; it keeps the lab safe for everyone involved.
Anyone who's ever worked in an organic synthesis lab knows how much a reliable supply chain matters for specialty reagents. Trifluoromethanesulphonic anhydride—often called triflic anhydride—pops up all over modern science, from pharmaceuticals to agrochemicals. Searching for it can feel tricky at first, especially because it falls under a group of highly reactive chemicals. Not every supplier will ship to every country, and purchase generally needs a business or institutional account.
In my own time in chemical research, I've had to source materials like triflic anhydride many times. Large chemical suppliers such as Sigma-Aldrich/Merck, Alfa Aesar, TCI, and Acros Organics routinely carry this reagent. For research groups tied to universities or established businesses, these suppliers often have agreements in place for direct purchase and delivery. To place an order, the company usually requires registration, documentation that proves scientific or industrial use, and a shipping address that can handle dangerous goods.
For boutique suppliers, Oakwood Chemical, Apollo Scientific, and SynQuest Laboratories also keep triflic anhydride stocked, but with the same requirements: proof of professional use and strong safety protocols. None of the major suppliers sell this compound to individuals. Online marketplaces might list trifluoromethanesulphonic anhydride here and there, but that path doesn't inspire much confidence—quality and legal shipment often take a back seat to fast sales.
The usual purity for trifluoromethanesulphonic anhydride from certified suppliers falls between 98% and 99%. That's plenty high for research or process chemistry. Lower purities would spell trouble, since reactive residues and water pick up in this compound can muck up sensitive reactions or even trigger side reactions that threaten worker safety.
I learned early on never to skimp on verifying a Certificate of Analysis. Good suppliers provide batch-specific purity data and show levels for typical impurities like hydrolyzed acid or trace metal content. Nobody wants to pay premium prices only to find out that a bottle contains more water than expected or an off ratio of the anhydride itself.
Fernand, a senior chemist I worked with, always said that “trusted supply lines pay for themselves.” Triflic anhydride won’t forgive shortcuts in handling, storage, or sourcing. Experienced suppliers ship under regulations that reduce accident risk. They include safety instructions, packaging rated for corrosives, and documentation that's not just paperwork, but insurance if regulators visit your premises.
Also, take regulations seriously. Laws in the US, EU, and Japan restrict shipment and purchase of triflic anhydride, considered both toxic and dangerous for environmental release. Skirting these puts both reputations and people in real danger. If your institution plans to buy, nominate someone to stay up-to-date with official chemical lists and required permits. I’ve watched entire projects stall when even experienced groups missed a change in import regulations.
For organizations outside the big research pockets, regional distributors sometimes source high-quality triflic anhydride directly from producers in China or India. Buyers need to check documentation: authenticity, registration details, transport safety measures, and an open channel for technical questions. Sometimes, a good distributor offers technical support in local languages, which ends up invaluable if you run a reaction at an awkward hour.
Purchasing trifluoromethanesulphonic anhydride calls for careful sourcing and a focus on purity. Labs grow stronger when they don't treat safety, paperwork, or quality as afterthoughts. The price tag represents not just a chemical, but peace of mind—knowing your science, your people, and your workplace are on solid footing.
| Names | |
| Preferred IUPAC name | Trifluoromethanesulfonic anhydride |
| Other names |
Triflic anhydride Trifluoromethanesulfonic anhydride Trifluoromethanesulfuric anhydride TFMSA Triflate anhydride |
| Pronunciation | /ˌtraɪˌflʊəroʊ.mɪˈθeɪnsʌlˌfɒnɪk ænˈhaɪdraɪd/ |
| Identifiers | |
| CAS Number | 358-23-6 |
| Beilstein Reference | 1201042 |
| ChEBI | CHEBI:40001 |
| ChEMBL | CHEMBL15441 |
| ChemSpider | 24113491 |
| DrugBank | DB14620 |
| ECHA InfoCard | 03d003db-b136-4e8f-943e-211a9c56306e |
| EC Number | 206-238-3 |
| Gmelin Reference | 89040 |
| KEGG | C14322 |
| MeSH | D019277 |
| PubChem CID | 6604211 |
| RTECS number | YP9625000 |
| UNII | 9B1B3N2EPL |
| UN number | “3265” |
| Properties | |
| Chemical formula | (CF3SO2)2O |
| Molar mass | 282.13 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Pungent |
| Density | 1.675 g/cm3 |
| Solubility in water | React violently with water |
| log P | -0.2 |
| Vapor pressure | 20 mmHg (20°C) |
| Acidity (pKa) | -5.2 |
| Basicity (pKb) | -12.3 |
| Magnetic susceptibility (χ) | -58.0E-6 cm³/mol |
| Refractive index (nD) | 1.333 |
| Viscosity | 6.8 cP (25°C) |
| Dipole moment | 3.68 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 383.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1326.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1565.4 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed, causes respiratory irritation, reacts violently with water. |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | Causes severe skin burns and eye damage. Causes serious eye damage. Harmful if swallowed. Harmful in contact with skin. Harmful if inhaled. |
| Precautionary statements | P261, P280, P304+P340, P305+P351+P338, P310, P405, P501 |
| NFPA 704 (fire diamond) | 3-0-2-W |
| Flash point | 42 °C |
| Autoignition temperature | 215 °C |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | Lethal dose or concentration: LD50 (oral, rat): 282 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2000 mg/kg (rat, oral) |
| NIOSH | TT4900000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not established |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Methanesulfonic anhydride Trifluoromethanesulfonic acid Methanesulfonic acid Trifluoromethanesulfonyl fluoride Trifluoromethanesulfonamide |