People often chase after stronger acids for both industrial and research work. Back in the mid-20th century, as chemists looked for ways to accelerate tough synthesis steps, the need for a tough, non-oxidizing acid grew. Trifluoromethanesulphonic acid—often called triflic acid—showed up on the scene as a heavy hitter. Early reports from the 1950s revealed it standing out compared to sulfuric acid. Chemists from industries like organofluorine started picking it out as a stronger and cleaner alternative, able to deliver results where older acids fell short. Over time, researchers dug deeper, uncovering tricks to make it reliably without busting budgets. The spread of triflic acid mapped onto the growing importance of tough acids in synthesizing silicones, fuels, and fluorinated compounds. Growth in R&D and industrial platforms kept triflic acid relevant year after year, ever since chemists first stumbled across its stubborn power.
Trifluoromethanesulphonic acid, known in labs as triflic acid, claims one of the highest spots on the acid strength charts. In simple terms, you see a clear, colorless liquid that carries a sharp, biting odor. Unlike more common acids, triflic acid doesn’t hold water—it drains right down stainless steel without showing much reaction. At room temperature, it pours like thin syrup, but once it hits a surface, it can scorch or corrode. Unlike fuming acids, triflic acid stands powerful and steady, not sending up choking clouds, so handling it under the hood makes lab work smoother for the most part. People working with fine chemicals and high-purity needs count on its no-nonsense behavior and robust chemistry to pull off reactions that stall with other acids.
If you boil it down, triflic acid’s story kicks off with its structure—CF3SO3H. Its trifluoromethyl group cranks up both acidity and stability, beating out sulfuric acid many times over. It melts down at around -40°C and boils close to 162°C. You’ll spot a density of about 1.7 g/cm3, which puts it among the heavier specialty acids. It mixes easily with organic solvents like acetonitrile, dichloromethane, or even dioxane, but it shrugs away from plain water. Tossing water into a beaker of triflic acid releases a burst of heat—reminiscent of the classic lesson: “add acid to water, not the other way around.” It’s less likely to oxidize materials, so oxidation-sensitive molecules and delicate organometallics stand a fair shot of making it through a reaction alive. The acid stays remarkably strong regardless of what it’s mixed with, ready to donate that proton every time.
On a container, you’ll read purity levels—usually 98-99%—and a reminder that this is one of the most powerful acids around. Triflic acid lands with a UN number for transport (UN 3265), full GHS labeling, and safety class ranging from skin corrosion to environmental warnings. There’s a sharp smell and warning for severe burns, so lab teams don’t let their guard down around it. Storage calls for cool, tightly sealed containers, preferably glass or PTFE-lined vessels. Labels spell out hazard pictograms—corrosive and health hazard—and instructions for keeping it away from incompatible materials like bases or active metals. Knowing which country or trade region you’re in changes the tune: EU, US, China, and Japan all stick to their strict rules, but the bottom line remains sticking to gloves, goggles, and clear ventilation.
Factories turn to several methods for making trifluoromethanesulphonic acid, but one technique stands out for its scale and cleanliness. One popular path runs from trifluoromethanesulfonyl chloride (CF3SO2Cl) and hydrolysis—start with a reaction between chlorosulfonic acid and trifluoromethane, then kick the chloride over to acid by treating it with water. Others skip over by using an electrochemical fluorination step, swapping hydrogen for fluorine atoms on methanesulphonic acid. Whichever path, the heart of the matter is clean, dry handling—the strength of the final product can tank with unintended water or metal contamination. The finished acid comes through purification steps—sometimes distillation under reduced pressure, other times filtration—until it streams into the glass bottles ready for shipping.
Anyone who’s tried acid-catalyzed reactions learns quickly that not every acid plays on the same level. Triflic acid shines in activating weakly reactive groups—think alkylations on hindered aromatics or tough ether cleavages. It outmuscles sulfuric and toluenesulphonic acids in both rate and yield. Put triflic acid in front of a dithiane, and it strips it with ease; introduce it to metal coordination complexes, and the acid motivates the ligand swap. Chemists cherish it for forming triflating agents—like triflic anhydride (CF3SO2)2O—that in turn power up even trickier transformations. There’s a knack for modifying the parent acid to spin off sulfonate esters or salts, too: swapping the proton for lithium or silver opens other doors in organometallic chemistry, especially in catalysis and radiolabeling. Its resilience takes catalysts into superacid territory, giving rise to those rare “magic acid” combos that settle long-standing arguments about aromaticity and carbocation stability.
Chemists toss around plenty of names for this acid. The IUPAC name reads out as trifluoromethanesulphonic acid, but short forms abound: triflic acid, CF3SO3H, TFMSA. Product catalogs from Sigma-Aldrich, Merck, and TCI flag batches with those very labels, but they all point at the same flask of colorless, heavy liquid. Sometimes, older texts refer to it as trifluoromethylsulfonic acid or call it simply fluoro-sulfonic acid, though that usually means something else in today’s catalogues. The shorthand “TfOH” shows up across patents, lab notebooks, and reference manuals, making life easier in fast-paced research settings.
No matter the setting, triflic acid draws extra respect for its set of hazards. Splash some on a glove and you’ll feel the sting through nitrile in no time—chemists and technicians who’ve been burned won’t soon forget. Polyethylene or neoprene gloves, face shields, and lab coats get dusted off before the first cap comes loose. Fume hoods keep fumes down, but spills need fast cleanup with neutralizing powder. Facilities keep eye wash stations nearby and teach everyone how to rinse acid from clothes and skin. Regular training drills focus on storage, with glass or PTFE containers kept cool and far from bases, reducing agents, and organic material. Teams review every move in writing before running a new process, catching the sort of single-mistake accidents that could put workers in a hospital. Transport teams follow DOT, ADR, or IATA protocols to the letter—nobody wants a truck full of triflic acid leaking on the highway.
Triflic acid stands tall in the lab and factory. In pharma, it turbo-charges Friedel-Crafts alkylations and helps make fragrance ingredients. In electronics, it carves out pathways for advanced etching and doping. Polymer scientists find its ability to dissolve tricky resins crucial for high-end membranes and fuel cells. Sometimes it even finds use in battery electrolyte manufacture, driven by the swelling demand for electric vehicles and clean energy systems. Catalysts based on triflic acid keep the pace up in petrochemical plants—especially where strong, non-oxidizing acid does the job with fewer side reactions. Studies into next-generation drugs and specialty chemicals often call for a reagent with muscle, and triflic acid keeps making the cut.
The research lab turns into a playground for triflic acid. Graduate students and postdocs target ever more complex molecules, using its unique strength to coax out products elusive to weaker acids. Historically, Nobel-level work has spun out from acids like triflic, settling debates about carbocation rearrangements and “superacid” chemistry. Industrial R&D outfits chase greener, safer preparation methods, cutting down by-products and energy use. Synthetic chemists optimize routes to high-value APIs and start materials, balancing cost against safety needs. Electrochemistry teams roll out prototypes using triflic acid as a proton conductor or membrane stabilizer, starting new conversations about the limits of fuel cell tech. Even work in nanochemistry leans on it for etching and surface preparation.
Toxicologists don’t give triflic acid a free pass. Regular contact brings skin and eye burns, while inhalation of vapor can damage airways. Cleaning up after spills takes real discipline—missing a spot risks late-night trip to the clinic. Lab animals exposed to dose ranges show acute corrosion and delayed healing. Still, it doesn’t build up in the environment the way some heavy metals do. The push now focuses on chronic exposure, seeking any signs of long-term trouble for industrial workers. Waste handling—neutralizing before discharge, safe storage, and dedicated containers—takes priority, aiming to keep run-off from mixing downstream. Governments follow the lead of regulatory science, with safety data sheets growing thicker every year as new findings become available.
A decade from now, expect even more visibility for triflic acid. Newer, cleaner manufacturing sites keep popping up, reflecting both increased demand and tighter environmental controls. Recycling methods inch closer to closing the loop for this high-value acid—lab-scale pilots start feeding into full production strategies. Academics tune its power for milder, greener chemistry, leading to “acid-on-demand” formats less likely to challenge worker health. Battery and fuel cell engineers tweak the recipe, seeking better ionic conductivity without corrosion. Regulatory agencies continue to shape labeling, transport, and disposal. The next breakthroughs in pharmaceuticals or energy storage may yet rely on a bottle of triflic acid and a tech who knows what they're doing.
Trifluoromethanesulphonic acid, known among chemists as triflic acid, earns respect in any lab dealing with powerful acids. Those in electronics or pharmaceuticals might regularly cross paths with this clear, almost ghostly liquid. Its reputation comes from being one of the strongest acids on the planet. Most common household acids can’t hold a candle to its strength—which means a little goes a long way in synthetic chemistry.
Every chemistry student learning the ropes hears about the value of acids that can push reactions forward, especially when competing reactions slow down the process. Triflic acid steps in here like few others. Drug companies rely on it to build complex molecules with precisely arranged atoms. The strength comes from the trifluoromethyl group, which pulls electrons away and ramps up reactivity. Everyday examples include painkillers, cancer treatments, and antiviral pills, where a single slip-up during synthesis can derail a valuable batch.
My own experience in research labs showed that a bit of triflic acid could convert a sluggish reaction into one that finishes in hours rather than days. Researchers find it easier to protect key parts of a molecule or swap out stubborn pieces using this acid, especially when alternatives fail.
Tech companies quietly depend on triflic acid, too. Large-scale chip makers have tight requirements for etching and cleaning silicone surfaces. Traditional acids can leave behind tiny contaminants. Triflic acid’s volatility makes it easy to remove completely after use, leading to fewer defects in chips and circuit boards. You find it in processes that help smartphones, laptops, and car sensors run faster and last longer.
Think about it: a single dirty batch can cost millions. Clean reactions mean stronger chips and longer phone battery life for everyone. Factories favor triflic acid since impurities drop dramatically with its use, and they can reclaim or neutralize it after the job’s done.
The strength of triflic acid also brings risks. Improper handling causes burns, and it can trigger violent reactions with water or organic substances. Labs use special glassware and ventilated cabinets just to manage the acid safely. If spilled, a drop on skin demands serious first aid right away. A bottle left uncapped fills the air with harsh fumes. The acid resists being broken down in nature, meaning trace amounts might stick around longer than intended.
Stricter rules and training can reduce accidents, but the most forward-thinking labs seek ways to limit or replace triflic acid. Green chemistry pushes for processes that need less hazardous materials or turn to solid acids and milder alternatives. Teams collaborate across universities and companies, inventing catalysts that can match triflic acid’s muscle without matching its risks.
Nobody doubts the importance of triflic acid in driving modern chemistry, especially where high yield and clean results matter. Still, safer handling and new technologies can reduce the dangers. My time in the lab taught me to never underestimate this acid—yet also showed how essential it is for medicines and microchips. Innovation keeps moving fast, and staying safe while doing world-changing chemistry feels more possible than ever.
Trifluoromethanesulphonic acid, usually called triflic acid, punches well above its weight in the lab. Chemists respect this clear, heavy liquid for the power it brings to reactions. That strength has a flipside: it wants to chew through skin, clothing, metal, and just about anything else not designed to withstand a true superacid. Coming face to face with it teaches the value of old-school safety, with no shortcuts.
One splash of triflic acid can mean instant, deep burns. It goes through gloves that pass for safe with most chemicals. Give it a chance and it eats metal bench tops and destroys glass on bad days. The fumes, even if invisible, can choke the lungs and leave a burning throat. The challenge extends beyond simple spills. Storage matters, venting matters, even removing the cap on a bottle demands respect for that hiss of vapor.
After seeing what can go wrong, no one I know skips proper gloves anymore. Forget standard latex—these melt before you even finish cleaning up. Thick butyl rubber or heavy-duty Viton gloves hold up better and keep hands safe. Splash goggles don’t cut it either. A full face shield stops accidental squirts from catching you off guard. Long sleeves, a flame-resistant lab coat, and a good chemical apron all close off the gaps that acids creep into.
Fumes travel further than expected. Working with triflic acid inside a properly running fume hood becomes second nature. You get used to closing the sash, checking airflow, and never reaching around with bare hands. The few who tried short cuts in my experience always regretted it. The smell lingers; so does the threat to lungs. Breathing gear rarely comes out, but stays close just in case of a spill outside the hood.
Leaving triflic acid on an open bench feels careless. Dedicated acid cabinets handle the risk. They keep bottles upright and control any leaks with strong metal trays at the bottom. Keeping it away from anything water-based avoids dangerous reactions. A slip-up mixing it with organic solvents or even wet paper causes violent releases of heat and toxic fumes. Even the tightest cap must get checked for crusts, corrosion, or dried-out seals.
Once spilled, triflic acid demands a clear head. Reaching for sodium bicarbonate seems natural, but the fizzing comes on wild and fast. Special acid neutralizing agents, designed for strong acids, get the job done with less risk of boiling and re-aerosolizing the acid. All spent gloves, wipes, and gear need bagging up and treating as hazardous waste. Letting small drops air dry puts everyone in the lab at risk, so a strict cleanup protocol is the rule, not a suggestion.
Nobody forgets their first encounter with triflic acid. Some chemicals let you drop your guard, but not this one. Regular safety drills, real training, and learning from near misses save skin and careers. New folks watch and learn before they touch the bottle. Safety data sheets sit on the bench, not unread gathering dust. Respect for triflic acid comes from the real stories—burns avoided, spills contained, and labs running another day without a hitch.
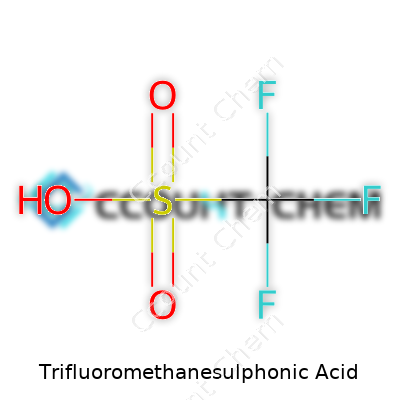
Standing in a laboratory, it’s easy to get lost in the technical names of chemicals. Trifluoromethanesulphonic acid, often shortened to triflic acid, goes by the formula CF3SO3H. Understanding that short string of elements opens a whole world of chemistry that plays a massive part in both industrial processes and the foundations of organic synthesis. For a compound with just one carbon, one sulfur, three fluorines, three oxygens, and one hydrogen, its personality fills a room.
This acid blows past the strengths of typical mineral acids like sulfuric or hydrochloric. That formula, CF3SO3H, describes one of the strongest known acids. It owes its strength to the way the trifluoromethyl group draws away electron density from the sulfur. The sulfonic acid group attached to those three fluorines transforms it into a sort of chemical sledgehammer. In practical terms, that means it can protonate almost anything, from water to hydrocarbons that normally laugh off acids.
I remember the first time I saw triflic acid used: it made even tough organic molecules bend and react. Chemists reach for CF3SO3H when regular acids fall short, like trying to force a reaction that refuses to move forward otherwise. One of its big jobs is activating or creating carbon bonds in pharmaceuticals, agrochemicals, and high performance materials. Factories rely on it to clean up reaction steps or to clean surfaces where nothing else works.
The raw power that makes triflic acid so attractive also demands careful respect. Its formula might seem simple, but it can eat right through glass and skin. Health authorities place strict controls on how it gets used, stored, and transported. Even small spills can start major accidents, so only trained folks with the right equipment go near the stuff. If someone ignores safety, the consequences are immediate and serious—anything from bad burns to chemical releases that harm more than just the user. Every time I worked around triflic acid, routines mattered—proper gloves, eye protection, well-ventilated hoods, and clear emergency plans.
The power of triflic acid brought huge benefits, but manufacturers and researchers keep searching for greener alternatives. Safer acids and solid catalysts mean less waste and lower risk, which cuts costs and health hazards. Some companies have rolled out reusable resin acids or switched to less aggressive chemicals where possible. Reducing reliance on strong acids like CF3SO3H isn’t just about ticking boxes—it’s about protecting workers and the environment while still keeping up advancements in medicine and technology.
Knowing triflic acid’s formula amounts to more than trivia. It guides how we use, store, and replace chemicals in the real world. Staying informed about what goes into the beaker—the elements, the strengths, the risks—serves as the first step toward safer labs, better products, and a smaller environmental footprint. Strong acids like this have earned their place, but every step we take toward safer and smarter substitutes improves both the lab bench and the communities that depend on those products.
Trifluoromethanesulphonic acid, often known as triflic acid, has a reputation for being tough on both people and materials. Chemists who handle it respect its strength. It’s one of those substances that doesn’t leave room for half measures. Its sheer acidity puts it in the same league as magic acid. Stories float around labs of glassware dissolving and gloves melting from a stray drop. Those stories remind you: any slip-up, and you pay the price—either with ruined equipment or worse, a trip to the emergency room.
In my experience, short cuts rarely pay off with chemicals like triflic acid. You need containers built for punishment. Only glass or PTFE (polytetrafluoroethylene) survive extended contact. Regular plastics go soft. Even stainless steel won’t guarantee protection, since the acid eats through most metals over time. At the university lab, we used thick-walled glass bottles, and even then, double sealing with PTFE-lined caps. It’s tempting to grab the closest thing at hand, but the peace of mind that comes from proper storage trumps any convenience.
Humidity ranks as a major enemy here. Water reacts instantly with triflic acid, blasting out fumes and generating even more heat. I’ve watched a colleague open a careless bottle—nothing dramatic happened, but minutes later, the acrid stench spread through two rooms. The bottle had sat with a loose cap under a leaky vent. Lesson learned: you store triflic acid in a dry, cool space, away from anything that leaks or sweats moisture. Even a forgotten sink nearby can introduce humidity you can’t see.
Direct sunlight speeds up degradation and can mess with labels or packaging. I always push chemical bottles deep into cabinets. The added darkness protects both contents and warning labels—faded ink can be as risky as a mistyped formula. Chemical fridges, or at the very least, ventilated cabinets, provide another layer of safety. A cabinet with a small exhaust fan works better than relying on a cracked window.
Some labs stack acids together. Mixing bottles to save shelf space feels risky. Triflic acid doesn’t play well with others—especially not with anything organic, bases, or oxidizers. Just a minor spill could start a chain reaction. I remember one incident: two acids sharing a shelf led to a slow drip and a gooey, hazardous mess. Clean-up took hours, but it could have been far worse.
A storage plan fails if people don’t stick to it. Too many accidents happen not because of ignorance but because someone ignores a protocol. Training new team members goes beyond a quick talk; hands-on walkthroughs and senior staff oversight make a difference. I’ve walked students through storage and handling, emphasizing that triflic acid can ruin more than just their experiment—it can set a whole project back.
Clear labeling, color-coded containers, and regular inventory checks reduce confusion. Dedicated acid cabinets with spill containment trays help catch drips before they spread. Keeping spill kits nearby, with proper instructions, lowers panic when things do go wrong. Digital tracking helps keep tabs on expiration dates and usage, so nothing sits forgotten.
Respect for chemicals, especially powerhouses like triflic acid, makes the difference between a safe lab and a disaster. Storing it right reflects a commitment to safety and responsibility for everyone who shares the workspace.
Trifluoromethanesulphonic acid, often called triflic acid, stands out for its strength as an acid. Anyone working with chemicals in a lab knows that some compounds are not just risky—they demand extra respect. Triflic acid lands near the top of my own mental checklist of acids that make you double-check your gloves and goggles before opening the bottle. Even seasoned chemists can trace scars or share stories about mishandling strong acids like this one.
The hazards start with direct contact. Spilling even a few drops on your skin can produce deep burns, much faster than you expect. If it touches your eyes, the damage can become permanent within seconds—a terrifying thought for anyone who values their sight. Inhaling even low amounts of vapors can corrode sensitive tissues in the nose, throat, and lungs. The acid’s strength comes from its ability to break molecular bonds, and the human body does not stand much of a chance against it without proper protection.
Another issue surfaces with its reaction to water. Triflic acid reacts violently with moisture, releasing heat and hazardous gas. Mixing it with the wrong solvent, or tipping over a bottle near a sink, can cause sprays or explosions. Most accidents don’t make the news, but they leave a lasting impact on those involved.
Quick thinking saves skin and lives. If you spill triflic acid on your skin, don’t wait—get moving. Take off contaminated clothing immediately and begin flushing the area with copious running water. Keep rinsing for at least 20 minutes. Forget home remedies. Soapy water helps, but plain running water remains the best tool.
Eye exposure demands absolute urgency. Someone needs to gently hold your eyelids open and keep water streaming across the eye without pause. Hospitals may use special eyewash stations, but if you’re without one, any source of clean running water works. After rinsing, go straight to a doctor or emergency room.
If you breathe in vapors, get outside to fresh air at once. Don’t try to tough it out if breathing feels odd or if you develop a persistent cough. Medical professionals need to assess the damage—lung and airway injuries often worsen over time. Swallowing triflic acid ranks among the most dangerous exposures, and seeking immediate help, without trying to make yourself vomit, remains the only reasonable course. Drinking water can dilute the substance, but medical attention cannot wait.
I remember working alongside new lab members who didn’t realize that splashes can land on exposed wrists or necks. Training goes beyond rules taped to the wall. Stories of real accidents and demonstrations of best practices engrain the seriousness of handling hazardous acids. The right gear—a face shield, splash-proof apron, heavy-duty gloves—makes the odds much better. Emergency showers and eyewash stations should not collect dust; they need to be checked and within reach. Clear safety protocols and a culture of caution cut down on injuries that change careers or lives.
Triflic acid has a place in chemistry, but its hazards should keep everyone vigilant. Preparing for accidents, knowing the signs of exposure, and acting without hesitation—these steps matter most. With honest communication and solid habits, the dangers lose some of their sting, and people can keep coming home in one piece.
| Names | |
| Preferred IUPAC name | trifluoromethanesulfonic acid |
| Other names |
Methanesulfonic acid, trifluoro- Trifluoromethanesulfonic acid Triflic acid Trifluoromethanesulfonic acid Trifluoromethanesulfonicacid TFMSA |
| Pronunciation | /ˌtraɪ.flʊə.rəʊ.miːˈθeɪn.sʌlˌfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 1493-13-6 |
| Beilstein Reference | 1209287 |
| ChEBI | CHEBI:27147 |
| ChEMBL | CHEMBL49978 |
| ChemSpider | 5799 |
| DrugBank | DB14206 |
| ECHA InfoCard | 100.003.412 |
| EC Number | 200-898-6 |
| Gmelin Reference | 82139 |
| KEGG | C01115 |
| MeSH | D014260 |
| PubChem CID | 6577 |
| RTECS number | TP4550000 |
| UNII | 9Q2A064K31 |
| UN number | UN2694 |
| Properties | |
| Chemical formula | CF3SO3H |
| Molar mass | 150.07 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Pungent |
| Density | 1.696 g/mL at 25 °C |
| Solubility in water | Miscible |
| log P | -2.1 |
| Vapor pressure | 14 hPa (20 °C) |
| Acidity (pKa) | -14 |
| Basicity (pKb) | -15.3 |
| Magnetic susceptibility (χ) | -40.0e-6 cm³/mol |
| Refractive index (nD) | 1.333 |
| Viscosity | 19 cP (25 °C) |
| Dipole moment | 1.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 364.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -803.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1418.4 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | J01MA23 |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. Causes serious eye damage. Toxic if swallowed. Toxic if inhaled. |
| GHS labelling | GHS05, GHS06, GHS08 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P260, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P363, P405, P501 |
| NFPA 704 (fire diamond) | 4-0-2-W |
| Flash point | 42 °C |
| Autoignition temperature | 400°C |
| Lethal dose or concentration | LD50 (oral, rat): 500 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 200 mg/kg |
| NIOSH | KL6300000 |
| PEL (Permissible) | PEL (Permissible): Not established |
| REL (Recommended) | 0.1 mg/m³ |
| IDLH (Immediate danger) | 30 mg/m3 |
| Related compounds | |
| Related compounds |
Methanesulfonic acid Perfluorooctanesulfonic acid Sulfuric acid Trifluoroacetic acid |