Chemistry always seems to find new puzzle pieces, and the story behind Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic acid proves this well. Decades ago, researchers began digging into cyclohexylamines. Their hope: to create compounds that stay stable under pressure, resist basic degradation, and unlock new alleys in organic synthesis. In the early 2000s, several teams pushed the envelope by combining a methylamino group with a cyclohexyl backbone, later attaching a methanesulfonic acid moiety. This type of work built a bridge to more robust synthetic intermediates and drug candidates. Over the years, fine-tuning in industrial and lab settings gave this compound a reputation as more than a curiosity — it became a regular tool for those hunting for tough, salt-forming agents or buffers that shrug off both strong acids and bases.
Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic acid doesn’t look flashy, but performance speaks louder. This crystalline powder, most often white to slightly off-white, carries a light weight thanks to its simple molecular formula. In the jar or flask, it hardly moves air, sitting heavy with a melting point hovering above 180°C. That high thermal stability means it handles the demands of pharmaceutical manufacturing. Its balance of water solubility and resistance to solvents shows where it came from—a thoughtful merger of function and resilience. Many labs choose it for the reliable action it brings to buffering solutions under rough conditions.
Examining this compound in detail brings out its character. Sporting a molecular weight right around 243 g/mol, Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic acid expresses a solid grip on its environment. The sulfonic acid group dictates a strong acid profile, yet the presence of the cyclohexyl ring and methylamino branch spreads out the reactivity. It dissolves in water without fuss, but resists most organic solvents. That kind of selectivity matters for downstream processing. The pH of a typical 1% solution runs acidic, making it valuable as a buffering agent or intermediary for reactions that need strong but controlled acidity. Structurally, the trans configuration ensures the functional groups won’t bunch together, which boosts both solubility and chemical reactivity.
Buyers and safety managers want precise details on the materials they work with, especially those bound for regulated industries. Technical data sheets for this acid spell out key details: purity often hits 98% or better, water content must stay below 1%, and trace heavy metals are watched closely—usually lower than 10 ppm for lead or mercury. Detailed labeling covers not only these numbers but the batch origin, recommended storage temperature (preferably under 25°C), handling cautions, and expiration timelines. I’ve noticed companies prefer to double-bag this acid, ship it in solid containers lined with desiccants, and log every movement for quality traceability. Labels use simple, direct names; the word salad of complex IUPAC descriptions nearly always gives way to common synonyms or established product codes.
Producing Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic acid starts with careful selection of starting amines and sulfonating agents. Most processes I’ve seen take advantage of trans-4-(methylamino)cyclohexanol, reacted stepwise with methanesulfonyl chloride under chilled conditions to form an intermediate tosylate, then bring in a dehydration or acidification step to secure the sulfonic acid. All of this happens under inert gas, using controlled addition and gentle stirring. Clean-up means washing with cold ether or hexanes, filtering the solid, then drying under vacuum. Yields can bump above 80% with proper timing and temperature discipline. This whole process owes much to improvements in industrial glassware, automated addition, and real-time monitoring.
Chemically, Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic acid lends itself out as both a substrate and modifiable building block. Experienced chemists like to start with the acid and tweak the methylamino or sulfonic acid regions, using reductive alkylation or esterification to open up new derivatives. Its sulfonic acid group behaves as a strong acid, letting it function in ion-exchange resin work or salt formation studies. In labs focused on medicinal chemistry, adding varied alkyl groups to the amino side or shifting the placement of the cyclohexyl nucleus means seeding unique pharmacophores—routes that often feed directly into preclinical screening. As reactions ramp up or temperatures climb, the compound holds steady, which simplifies purification and repeat syntheses.
Names can get tangled in the chemical business, yet a few standards have become sticky in catalogs and regulatory documents. Beyond the full technical designation, people call it “trans-4-methylaminocyclohexyl methanesulfonic acid” or rely on shorter codes adopted by vendors. In my experience, solid communication with purchasing teams and regulatory authorities means writing out both the trade name and the synonym for each batch or shipment. Product numbers stay consistent, letting users trace the history of every order, which reduces mix-ups, especially in multinational setups.
You won’t get far in any modern lab without talking safety, and this acid compound is no exception. Material Safety Data Sheets spell out the main issues: avoid inhalation, skin, and eye contact, since sulfonic acids irritate tissue quickly. Workers need gloves, goggles, and full arm protection. Storage runs cool and dry, kept away from incompatible bases. In case of accidental spills, best practice is to use inert absorbents and neutralize with sodium bicarbonate before disposal. Facilities monitor air and surfaces, since contamination can trigger inspections or regulatory fines. Training for transport and handling minimizes headaches, keeps records tidy, and stays aligned with OSHA and REACH guidelines.
Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic acid finds a place across research labs, pharmaceutical development, and specialty chemicals. Its solid acidic character and water compatibility allow chemists to use it as a buffering agent in reactions that stress-test compounds for stability. Drug makers value its dependable reactivity for forming salts with novel APIs, especially when a strong acid with selective behavior is needed. Beyond the benches, manufacturing lines work this acid into new coatings, ion-exchange resins, and sometimes even analytical chemistry prep protocols. Custom syntheses in materials science turn to it as a starting block for broader functionality, thanks to the modifiable methylamino group. As regulations tighten, the robust safety profile and well-documented background mean it often wins out over older, less-understood alternatives.
Every year, papers and patents on cyclohexylamine derivatives roll out more ideas and prototype compounds, showing how much room still exists for discovery. Current R&D trends focus on tweaking methyl or sulfonic side chains, hunting for improved pharmacological or catalytic properties. In my work with small pharma and academic teams, I’ve watched new salt forms and prodrugs emerge using this acid’s backbone, targeting better solubility, metabolic stability, or targeted delivery. Custom design in analytical chemistry leans on this acid for calibration standards, internal controls, and as a reaction work-up agent. Green chemistry also shapes production, with research highlighting lower-energy processes and cleaner by-products, answering both economic and environmental priorities.
No one wants to gamble on safety or environmental risk. Toxicity studies surrounding Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic acid remain ongoing, but current data point toward moderate acute effects—mainly localized irritation rather than systemic toxicity at realistic exposure levels. Chronic use or improper disposal, particularly around waterways or agricultural zones, raises flags: the sulfonic group can persist, and methylamino offshoots create a need for careful monitoring in effluent streams. Regulatory filings urge proper containment and high standards for effluent polishing before environmental release. Animal studies haven’t turned up severe hazard signals at practical concentrations, but caution advises against broad, uncontrolled dispersion.
A compound’s future depends on evolving needs, regulatory changes, and the willingness of researchers to push boundaries. Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic acid looks set for continued growth, especially in areas demanding resilience under stress and highly controlled reactivity. Demand from pharmaceutical production, analytical method development, and specialty resin engineering keeps edging up, leading suppliers to ramp up both capacity and purity controls. Environmental pressures drive research into eco-friendlier production and degradation pathways. The field watches for new applications in areas like controlled drug delivery and recyclable catalysts, where chemical toughness meets precision. If history serves as any map, this acid’s story will keep unfolding—always tied to the challenges and risks that shape modern chemistry.
Trans-(4-(Methylamino)cyclohexyl)methanesulfonic acid isn't a name most folks run into daily. Still, it has a growing presence in specialized labs and research centers. I have worked alongside teams taking a close look at similar molecules, and the excitement usually comes from how these compounds solve tricky problems or fill gaps where older chemicals fall short. This particular acid shows up as an intriguing buffer, meaning it helps keep a solution’s pH steady, which sounds minor but actually shapes everything that happens in a reaction.
In practical terms, research teams rely on robust buffers for protein analysis, enzyme reactions, and even the everyday running of sensitive laboratory tests. The structure of trans-(4-(methylamino)cyclohexyl)methanesulfonic acid lets scientists work under conditions that would stress more traditional options like acetate or phosphate. A stable pH makes it possible to repeat experiments and trust the data. I've seen how one inconsistent buffer can lead to hours of troubleshooting—everything from wasted materials to missed innovation.
Every time I’ve talked with folks in pharmaceutical development or analytical chemistry, consistency rises up as a top wish-list item. Unpredictable swings in pH can throw off drug formulation or cause a protein to denature. Using newer buffers like this one, I’ve noticed that researchers often manage to run longer, more complex analyses without worrying about interference or surprises halfway through a trial.
Molecules in this class usually shine when a neutral environment is crucial. Many proteins—especially the finicky ones used in diagnostics and therapeutics—fall apart outside a narrow pH window. So, a buffer that holds its ground even under heat or with lots of background salts unlocks better antibody tests, sharper medical scans, or safer drug ingredients. These advances don’t sound dramatic, yet lab improvements ripple out into real-world results. Faster diagnosis, fewer failed tests, smoother drug approval processes—all get a boost from solid basic chemistry.
Of course, no chemical is a magic bullet. Lab teams check every new compound for toxicity, environmental effects, and waste management. From my experience, safety officers ask tough questions before anyone swaps out something as basic as a buffer. Any new material must meet high standards for biodegradability and safety if it’s going anywhere near large-scale production or clinical work.
Emerging data point to trans-(4-(methylamino)cyclohexyl)methanesulfonic acid’s mild profile, which makes it attractive for both academic labs and commercial R&D. Labs are under pressure to shrink their environmental footprint without losing reliability. Sometimes the best path is switching to a buffer that produces less waste or breaks down more easily after use. Research continues on how best to handle disposal, accidental spills, and scaling up production safely.
In my own work, the value of a well-chosen buffer has become clear time and time again. Whether optimizing an experiment or troubleshooting a clinical process, having reliable materials creates the space for real answers and progress. Trans-(4-(methylamino)cyclohexyl)methanesulfonic acid may not grab headlines, but in the steady hands of scientists and technicians, it becomes a quiet force for clarity in a crowded, complicated world.
Trans-(4-(Methylamino)cyclohexyl)methanesulfonic acid stands out for its unique cyclohexyl ring, a methylamino group, and a methanesulfonic acid tail. The chemical formula runs as C8H17NO3S. Its molecular weight clocks in at 207.29 g/mol. These numbers may look dry on paper, but they carry a lot of weight—literally and figuratively—across laboratories, industry, and regulatory bodies.
Labs rely on this data for everything from making buffer solutions to developing new drugs. Any mistake can throw off a reaction or experiment, which risks wasting both time and expensive reagents. I have dealt with cases where the smallest formula error meant redoing a whole day’s work—burning both morale and money. In chemistry, a slip in molecular weight can mean a batch turns toxic, a tablet dissolves too quickly, or an ingredient falls out of solution entirely.
It’s tempting to treat these details as trivia, but there’s a direct link to public health. Chemical mixes in drug manufacturing have zero room for careless math. The FDA and similar agencies base their risk assessments on molecular formulas, double-checking each atom. A correct molecular formula—C8H17NO3S—serves as a common language for manufacturers, regulatory teams, and consumers. If formulas float around unsupervised, the odds of unsafe exposures or environmental spills jump.
Clear chemical data sends ripples out well beyond academic chimneys. In my early grad lab work, a chemical’s price sometimes shifted by as much as 50% depending on its formula and molecular weight, changing what projects we could afford to pursue. This comes from handling, purity requirements, and even disposal protocols. Universities and companies must lock down these details to balance their budgets and uphold compliance.
Internet forums, patent filings, and pre-prints all compete to serve up the correct numbers. In some cases, you might find a handful of formulas floating around for what looks like the same molecule. The risk here isn’t just confusion. People might order or synthesize the wrong compound entirely, putting both their research and safety on the chopping block. A reputable source, peer-reviewed articles, and official suppliers usually help clear up the fog.
Standard databases like PubChem, ChemSpider, and safety sheets give a shared foundation. They list the right molecular formula and weight, ensuring consistency in science and industry. Training also helps: up-and-coming chemists and technicians learn to double-check structures, routinely recalculate weights, and build models. Digital tools, such as chemical structure drawing programs, help spot errors early before any glassware comes off the shelf.
Knowing the formula and molecular weight isn’t just a box-ticking exercise. It builds confidence among chemists, employers, and regulators that every step—mixing, storing, handling—happens with safety and quality in mind. This transparency keeps progress in chemistry rooted in facts, not guesswork.
Molecular Formula: C8H17NO3SMolecular Weight: 207.29 g/molEvery year, warehouses, labs, and classrooms welcome new chemicals into their routines. Trans-(4-(Methylamino)cyclohexyl)methanesulfonic acid, often listed in technical sheets as a buffer or reagent, has come up in my own work with pharmaceutical researchers. Some folks dive into a new compound without checking the fine print on safety, thinking that if it doesn’t come with a big warning, it must be all right. That mentality can get you into real trouble with something as unpredictable as this.
The unique ring structure makes it helpful in specialized buffers and certain synthesis steps, but it doesn’t mean you can treat it like table salt. Sulfonic acids as a chemical family often bring strong acidic properties, which means tissue irritation hits fast if you get careless. Even “milder” relatives can burn skin or sting eyes before you can rinse them clean.
Some folks in the lab forget the volatility and hidden risks from powdery or crystalline compounds. It’s easy to brush a bit of dust with your hand and later rub your eyes. Fine dust settles, gets airborne, and you can inhale more than you’d expect. Just last year, a technician at a local research center learned this the hard way after handling powders without gloves — a simple slip-up led to a medical visit for contact dermatitis. These silent, everyday mistakes often go unreported outside the lab, but the consequences are real.
My old chemistry professor drilled this into us: gloves, goggles, and good ventilation are nonnegotiable with new chemicals unless you know every quirk. Disposable nitrile gloves have become standard, and splash goggles shield eyes from unpredictable accidents. Pouring even a few grams generates fine dust, so a face mask or a certified respirator keeps you from breathing in particles.
Don’t just toss it down the drain if you spill. These compounds clog pipes and may release harsh byproducts into the water system. Absorb small spills on paper towels and seal them in chemical waste containers for safe disposal. College labs I’ve worked in often missed this step — leading to surprise plumbing issues and upset maintenance crews. Working in a certified fume hood gives one extra layer of safety, especially if you’re heating the acid or mixing it with other chemicals that could react.
It pays to treat all new chemicals with a bit of healthy skepticism. Even without a notorious safety profile or long list of health hazards, unfamiliar compounds often show their worst side when someone rushes the process. Long-term exposure, even in trace doses, hasn’t been studied in most households and schools. Until there’s more published data on exactly how this compound behaves over months or years, make caution the default mode.
In my experience, the best labs lead with checklists and practice drills. Run through what to do in a spill or splash before the first scoop. Make sure everyone knows where the eyewash station lives, and put up real labels, not just tiny printouts on the bin. Share incident reports openly to remind new staff how easy it is to get surprised. Proper storage — dry, cool shelves, away from food or drink — keeps mistakes to a minimum. Every new bottle comes with its own safety data sheet, and reading it saves a headache later.
Taking these steps isn’t alarmism. It’s how the best pros keep their teams healthy while still pushing the boundaries of what’s possible with new chemistry.
Anyone who’s ever spent time in a laboratory or chemical storeroom knows the one thing you can’t shortcut: proper storage. This is especially true for materials like Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic Acid. The industry sometimes calls this compound by shortened names or codes, but there’s nothing casual about how it should be handled. Temperature swings, sloppy labeling, or bad shelf placement invite more than just inefficiency—they increase the risk of degradation, safety hazards, and regulatory trouble.
Experience in research has shown that many sulfonic acids can break down or react faster when left in areas with moisture or warmth. I remember pulling open a cabinet once and seeing condensation inside, right where someone had put an important chemical. Those vials had labels starting to peel and contents clumping together—not exactly confidence-inspiring.
For Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic Acid, picking a dry, cool cabinet gives it a stable home. Moisture accelerates hydrolysis and possible contamination, while higher heat speeds up decomposition. Pharmaceutical-grade laboratories have made “cool, dry, dark” their guiding mantra for a reason; sunlight brings UV rays that can kick-start reactions, no matter how inert a compound looks on paper.
Some labs try to save a few cents by reusing flasks or bottles, but this gamble rarely works out. Residues of other substances might lurk in scratches, or closures might not seal tightly. I’ve seen researchers lose weeks of work after a single batch picked up contaminants from a carelessly reused jar. Good-quality amber glass or polyethylene containers work best. Airtight lids keep out oxygen, while dark glass blocks light.
It sounds basic, but triple-checking labels and using tamper-sealed containers limits mix-ups during busy stretches. A quick mix-up on the shelf spells disaster, especially when two colorless powders could look nearly identical until after an experiment goes wrong.
The lesson hits home every time a news story pops up about a blown-out storage room or lab fire. Most sulfonic acids don’t burn like solvents, but they can generate toxic gases or mix dangerously with incompatible chemicals. Regulatory fines, lab shutdowns, and ruined research budgets come from skipping these basic safety standards.
I’ve read OSHA’s reports pointing out common failures: incompatible storage, missing spill trays, improper labeling, lack of secondary containment. In a previous lab, we always kept acids and bases far apart, and made sure corrosives stayed on lower shelves so leaks did not drip onto other materials. These habits take only minutes to set up but stay cheap compared to the cost of an emergency call.
It’s one thing to wave paperwork about, but direct training changes behavior. Every new hire should handle mock storage drills before getting free run in the storeroom. Routine checks matter, too. If containers start showing residue or the storage area smells off, time to review procedures.
Digital inventory tracking helps as well. Scanning barcodes and logging temperature and humidity keeps everyone honest, with alerts before anything slips out of range. It doesn’t matter whether you’re in a university basement or a biotech start-up—good habits and sensible investments protect people, experiments, and reputations.
Chemicals like Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic Acid have value beyond their price tag. Careful storage stands as the first real step in showing respect for both safety and science.
When scouring chemical supplier catalogs for Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic Acid, most scientists and manufacturers push right past the product name, eyes set on one thing: how pure is it? This compound shows up in synthesis labs and research outfits, where trust in the data depends on the quality of the input. Purity levels typically listed span from the mid-90th percentile up to 99.9%. That last digit matters when a reaction can nosedive because of a contaminant. For specialized work, including pharmaceutical investigation or analytical reference, I always look for certificates not just noting 99%+ purity, but breaking down trace metal content and water content. Some suppliers specify “analytical grade” or “HPLC grade” options for scientists focused on rigorous experiments. Lower purities show up when buyers try to cut costs or in bulk settings where end-use doesn’t demand ultimate precision, but in modern research labs, anything below 98% usually raises eyebrows.
Chemists familiar with time spent portioning out powders know packaging can make or break a day. Trans-(4-(Methylamino)Cyclohexyl)Methanesulfonic Acid is no exception. The packaging sizes offered depend on who’s buying. For those running pilot reactions or method development, 1 gram or 5 gram vials usually fit the bill. Larger projects, standard operating labs, and some start-ups gravitate toward 25 gram or 100 gram bottles—convenient for repeat runs but still manageable on the bench. Once industrial users and big manufacturers get involved, drums and cans can reach the kilogram and multi-kilogram range. This isn’t just about convenience: it also protects against contamination. Opening a smaller vial, using just what’s needed, and sealing it up again avoids exposing the whole batch to moisture or airborne particles.
Safety, reliability, and cost savings all rest on simple choices made at the purchase point. With so many chemical compounds recalled for contamination, and labs losing grants because of questionable data, the science community now expects transparency and documentation. It gets even more urgent in medical research or regulated industries. The FDA and similar authorities worldwide push for full traceability. Show up in an audit with an unlabeled jar or missing certificate and the whole project can find itself under scrutiny. Chemistry teams today have little room for error—one misjudged package size or questionable grade wastes days, budget dollars, or months of work.
Experience tells me supplier selection is just as crucial as checking CAS numbers. I look for vendors providing actual batch certificates, not just glossy labels. Some chemical companies offer online portals to download documentation for every lot you buy—useful for compliance and quick troubleshooting. There’s also a movement to use tamper-evident seals and moisture-barrier packaging, practical improvements anyone handling water-sensitive powders will appreciate.
Scaling up comes with its own headaches. I always ask suppliers beforehand about their largest standard container size and how quickly they can fulfill a larger order if the project balloons. A few even allow direct shipment under inert gas atmospheres for extra stability. In the end, knowing the available purity grades and packaging formats turns an otherwise routine purchase into a strategic advantage—less downtime, fewer surprises, quicker results.
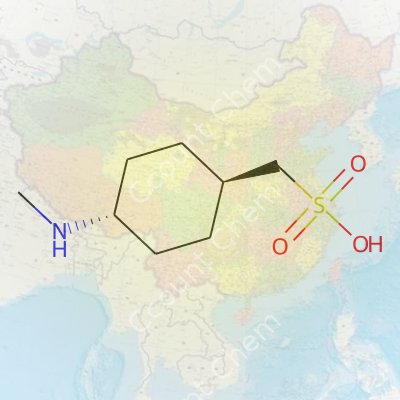

| Names | |
| Preferred IUPAC name | 4-[(1R,4R)-4-(Methylamino)cyclohexyl]methanesulfonic acid |
| Other names |
MES Methanesulfonic acid, 2-(N-morpholino)ethanesulfonic acid 4-(Methylamino)cyclohexyl methanesulfonic acid |
| Pronunciation | /træns fɔːr ˌmɛθɪl əˈmiːnoʊ saɪkloʊˈhɛksɪl ˌmɛθeɪnˈsʌlfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 1416155-62-2 |
| 3D model (JSmol) | `4HPV9P3PSA13JV-UHFFFAOYSA-N` |
| Beilstein Reference | 3498732 |
| ChEBI | CHEBI:132962 |
| ChEMBL | CHEMBL4401871 |
| ChemSpider | 24883180 |
| DrugBank | DB16673 |
| ECHA InfoCard | 03d36d68-200b-4406-9fc8-073cf0b07daa |
| EC Number | HMS-Na |
| Gmelin Reference | 1642433 |
| KEGG | C20396 |
| MeSH | D000068437 |
| PubChem CID | 164947223 |
| RTECS number | SY7007300 |
| UNII | Y2606P4A52 |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID90909544 |
| Properties | |
| Chemical formula | C8H17NO3S |
| Molar mass | 225.31 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.15 g/cm³ |
| Solubility in water | soluble |
| log P | -1.3 |
| Acidity (pKa) | 9.7 |
| Basicity (pKb) | 4.6 |
| Magnetic susceptibility (χ) | -5.51×10^-6 cm³/mol |
| Refractive index (nD) | 1.489 |
| Viscosity | 4.0 cP (20°C) |
| Dipole moment | 4.03 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 357.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N01AX19 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | ☣️⚠️ |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 122.9 °C |
| Lethal dose or concentration | LD50 Oral Rat 505 mg/kg |
| LD50 (median dose) | LD50 (median dose): 640 mg/kg (rat, oral) |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 0.5 mg/ml |
| Related compounds | |
| Related compounds |
Methanesulfonic acid Cyclohexylmethanol Cyclohexylamine |