Toluene-4-sulphonohydrazide first found attention among chemists in the 1960s, a time when organic chemical research aimed to open up options for safe and efficient industrial blowing agents. This compound presented an attractive balance: strong gas-evolving properties without handling headaches seen with other agents. Early manufacturing relied on sulfonation of toluene, followed by conversion to the hydrazide, and the material quickly earned its keep in the production of expanded plastics. As environmental regulations shifted after the 1980s, processes sharpened to reduce byproducts and toxic intermediates. Academic and industrial teams published improved syntheses, some of which lost the need for harsh conditions or corrosive reagents. These improvements meant lower emissions and made the substance more viable in markets headed for tighter chemical safety regulations.
This organic compound belongs to the hydrazide family, created from p-toluenesulfonic acid. In practice, toluene-4-sulphonohydrazide offers predictable thermal decomposition and reliable gas output, leading to widespread use where stable blowing action matters. I remember the days in rubber testing labs when batches of polymer foam differed wildly depending on the foaming agent used. Switches to this hydrazide often cleared up those problems due to its softer, more controlled gas release curve. It stands as an off-white powder or crystalline solid, with a mild to moderate odor, cleaner than many alternatives. Its compatibility with PVC and polyolefins brought it into the sphere of modern foams, microcellular plastics, and even expanded thermoplastic rubbers.
Every technician reaching for a bag of toluene-4-sulphonohydrazide notices its fine crystalline texture and lack of significant dusting, welcome in busy compounding rooms. Uncoated, it melts between 137°C and 141°C and begins decomposing slightly above this range with evolution of nitrogen and other gases. The compound’s stability below use temperature simplifies storage and extends shelf life, two crucial factors in plant environments where ambient temperature swings can wreak havoc on less stable materials. Water solubility runs low, making it unlikely to swell or clump in humid areas, and it dissolves better in polar organic solvents, a trait utilized in selected modification steps. Chemically, the hydrazide group reacts with oxidants and certain acids, but shows resilience with standard stabilizers.
Industrial-grade material comes with stringent assay requirements, generally over 98% purity by HPLC or GC, and tightly managed moisture content, because residual water influences decomposition on heating. Labels list the chemical formula C7H10N2O2S and the CAS number 80-51-3. Regulatory declarations often require explicit mention of dust potential, decomposition gases, and limitations for end-use—especially in food-contact products. My conversations with regulatory compliance officers revealed their focus on batch traceability, so good manufacturers print clear batch codes, date of manufacture, and safety pictograms. Handling recommendations align with those found on similar nitrogen-based blowing agents: proper ventilation and the use of personal protective equipment.
The craft begins with sulfonating toluene, producing p-toluenesulfonic acid. This intermediate reacts with hydrazine hydrate in polar solvents like ethanol or water. In older patents, the temperature holds near room conditions, but modern advances use controlled addition to limit side reactions and bump yields. Post-reaction, crystallization followed by filtration and washing removes excess hydrazine and mother liquor impurities. Laboratories I’ve visited use vacuum drying at 40–50°C to fully remove solvent residues. Alternative syntheses rely less on concentrated acids and push toward minimal byproduct formation—a nod to stricter chemical waste regulations of today.
Toluene-4-sulphonohydrazide acts as a modest nucleophile, reacting mainly under heat or catalysis. It decomposes predictably at elevated temperatures, making it reliable for use in foam creation. Extensions into custom substituted derivatives leverage the aromatic ring, allowing introduction of halogens or alkyls to tune decomposition temperature or modify gas output for special foam characteristics. Some research groups introduced stabilizers directly onto the molecule, aiming to extend utility into higher temperature polymers. In most modification reactions, steric hindrance around the hydrazide limits aggressive substitution, a tradeoff for its thermal reliability in commercial products.
Anyone searching chemical supplier catalogs might find this compound under names like p-toluenesulphonyl hydrazide, TSNH, or simply TSH. Commercial listings in North America sometimes carry trade names such as "Celogen TSH" or "BlowFoam-H," pointing to its tailored application for foaming. Avoiding confusion between these and unrelated sulphonyl hydrazides demands careful attention—shared synonyms across languages and regulatory lists often trip up even experienced purchasers and brand managers in global supply chains.
Worker exposure always stands as the core safety issue with hydrazide-based blowing agents. Acute toxicity runs lower than classic azodicarbonamide or benzene derivatives, reducing emergency incidents, but the usual PPE—nitrile gloves, goggles, long sleeves—remains non-negotiable. Adequate ventilation prevents buildup of airborne dust and nitrogenous off-gassing, which can irritate airways. Storage away from oxidizers, acids, and ignition sources ranks high on the list. At plants I have toured, rigid protocols require all spills go through well-documented decontamination and waste handling. Transport falls under dangerous goods guidelines due to the potential for decomposition and self-heating in bulk. Fire suppression planning includes foam or dry chemical agents—water only when fire involves non-confined spills. Safety data sheets advise first responders about symptoms of acute exposure and the correct steps for containment.
Commercial markets pull in toluene-4-sulphonohydrazide wherever finely tuned foam production drives value. PVC shoe soles called for it to get soft, consistent texture that didn’t yellow or stiffen in sunlight. Non-toxic gas output made it a lead candidate for children’s toys, sport flooring, and underlay mats. Thermoplastic elastomers benefit from its clean decomposition profile, avoiding residue that clogs extruder dies or damages clarity. Rubber manufacturers blend this hydrazide for closed-cell structures used in gaskets, seals, and noise-damping panels in automobiles. Its high purity and low odor open up medical-grade foaming and precision microporous plastics. Experimental coatings and even some bioplastics look to it for reliable void formation without generating toxic breakdown products. Across every application, processors count on predictable expansion and the absence of persistent organic pollutants.
Fresh research drives the story forward for this compound. Scientists in universities and corporate labs keep refining synthesis processes to squeeze out waste and cut down hazardous reagents. Nano-structured versions, sometimes stabilized with silica or alumina, show promise in reducing minimum activation energy for blowing—translating into finer, more controlled foam bubbles. I’ve seen industry consortia share findings about blends with organic peroxides and trial new stabilizers, all aimed at bringing the chemistry into compliance with emerging green chemistry frameworks. Biodegradable packaging sectors hope to exploit custom hydrazide derivatives to launch new foams that degrade on purpose, rather than persisting in landfills. Academic articles explore surface modification techniques to boost compatibility with novel polymers, and advanced analytical methods continue to sharpen the picture of decomposition product profiles.
The toxicity story begins with repeated lab animal assays and stretches through to in-vitro tests of metabolites. Available data suggest acute oral and dermal toxicity rates that fall below regulatory concern levels for short-term exposures. Chronic toxicity has barely any evidence of carcinogenicity, though regulators continue annual reviews in light of ever-evolving chemical safety standards. Lab studies show breakdown products (mainly nitrogen and toluene moieties) clear quickly from the environment, with low bioaccumulation markers. In tightly regulated product segments—food contact materials and children’s toys—manufacturers must provide certificates indicating extractable monomer and decomposition product levels below established health thresholds. Even as risk remains low compared to some legacy substances, chemical stewardship increasingly drives manufacturers to pursue improvements in purity, reductions in batch-to-batch impurity variation, and better occupational hygiene.
Looking ahead, toluene-4-sulphonohydrazide faces pressure to adapt or be replaced. Digital production and quality systems demand zero-defect, tightly monitored additives, and this compound sits at the intersection of tradition and ongoing innovation. Green chemistry regulations keep tightening, nudging producers to drop harsh acids and excess solvent in the upstream synthesis. Specialty foams and novel biodegradable plastics pull researchers into designing hydrazide-based agents with decomposition tailored for new resin chemistries. Automating quality control brings down costs in high-volume plants, but only when the blowing agent upholds its side of the stability bargain. Applications in wearable devices, soft robotics, and medical foams bank on even tighter controls over purity and emission profiles. Economic incentives and evolving workplace safety standards will continue to shape research priorities, with the end goal to meet market demand for safer, cleaner, and more adaptable foam solutions.
Most people have not heard of toluene-4-sulphonohydrazide, but if you’ve used anything made of foamed plastic, you’ve likely benefited from it. This chemical shows up in production lines that create everything from shoe soles to insulation panels. The trick to modern plastics often comes down to blowing agents—compounds that create bubbles and pockets in the plastic, making it lighter, softer, or better at absorbing shocks. Toluene-4-sulphonohydrazide helps produce these effects as a blowing agent, especially in PVC (polyvinyl chloride) and some rubbers.
The key trait of toluene-4-sulphonohydrazide lies in its ability to release gas when heated. During the process of heating plastics or rubbers, this substance reacts and lets off nitrogen and other gases in a controlled way. As the plastic heats up and the gas gets channeled in tiny bubbles, the final material ends up lighter and usually much better at cushioning. Take yoga mats, for example—those soft, spongy feels come in part from blowing agents like this. In my time working with production data for sports goods, I noticed just how carefully manufacturers tweak the contents of their foam to hit the right density and bounce.
There’s another side to this chemical’s use. Shoe makers turn to toluene-4-sulphonohydrazide for midsoles and insoles where a careful balance of structure and softness matters to prevent sore feet. The automotive industry uses it in lightweight panels, which helps shave some weight off vehicles. The reason? Lighter cars burn less fuel—a clear win for everyone.
Working with toluene-4-sulphonohydrazide brings up questions about safety and health. I’ve seen factory setups where protective gear and proper ventilation stand as top priorities. If not handled right, some of the gases formed during the heating stage could irritate eyes or skin, or cause breathing troubles. Regulatory agencies set exposure limits and call for good handling habits, but there’s a push for greener and even safer alternatives.
A thorough study by the European Chemicals Agency shows that exposure risks fall when good controls stay in place, but safety always depends on companies training crews well and monitoring air quality. There’s no getting away from the fact that blowing agents supply an answer for material performance, but the process asks for responsibility and regular checks.
Research teams are hunting for new ways of foaming plastics without the drawbacks tied to older chemicals. Already, some manufacturers use water-blown foams or switch to compounds that break down with less risk to the workplace and the wider world. Governments and NGOs encourage this shift because some agents can linger in the environment. Manufacturers face tight deadlines to meet new green standards without giving up on function or price.
The story of toluene-4-sulphonohydrazide covers a lot of ground. It serves a practical, even essential function in industries as different as footwear and auto parts, but also fronts up to the challenge of safety and sustainability. Better methods, safer chemicals, and clear rules can help production lines stay strong without putting health on the back burner.
Toluene-4-sulphonohydrazide, known in the chemical world as C7H10N2O2S, might seem obscure to those outside plastics or rubber manufacturing, but its signature—seven carbons, ten hydrogens, two nitrogens, two oxygens, and a sulfur—tells a big story. Familiarity with that formula brings more than trivia; it allows safety officers, technical developers, and even consumer advocates to pin down its behavior in the complex jungle of chemicals found in industrial settings.
Most people run into toluene-4-sulphonohydrazide without even knowing it, especially in products relying on expanded or foamed plastics. Factories use this compound as a blowing agent for PVC, EVA, and rubber. In real terms, it helps create the cushioning you put in your shoes or the padding in your yoga mat. As someone who’s walked through factory floors and felt the pressure of making safe, durable products on tight timelines, clarity about chemical building blocks like this one builds trust with purchasers and regulators.
Knowing that formula also means recognizing how the compound behaves. The two nitrogens flag the hydrazide group, notorious for releasing nitrogen gas on heating—key for making those tiny air pockets in foam. Safety managers depend on this quantitative view; one missed calculation could raise the risk of unwanted side effects, both in the product and on the factory floor.
Public health experts might not debate molecular formulas every day, but they do live and breathe their consequences. With increased scrutiny on occupational health risks, every small detail matters. The sulfur in the mix nods to the possibility of sulfur dioxide if mishandled at high temperatures. Prolonged or careless exposure can mean headaches, irritation, or worse. It never hurts to revisit material safety data sheets, double-check exhaust ventilation systems, or make sure batch records capture every detail about additives.
Transparency here protects more than workers; downstream users—people who cut, heat, or repurpose end products—also need assurance that by-products and degradation products stay within safe limits. A friend working in product safety once shared how overlooked residues from foaming agents led to costly recalls after unexpected emissions cropped up during use.
Rules and regulations continue to shape which chemicals get used and how. Almost every jurisdiction keeps tight tabs on substances with the potential for harm or environmental persistence. Toluene-4-sulphonohydrazide faces compliance checks under frameworks such as REACH in Europe and Toxic Substances Control Act in the U.S. Lawmakers push for disclosure and, in some cases, safer alternatives with less volatile decomposition products.
Chemists now engineer alternatives, aiming to lower toxicity while preserving foam quality. Sometimes this involves tweaking the structure to add more environmentally friendly groups or reduce nitrogen content, which can cut down on hazardous emissions. The process is far from easy, often requiring tough tradeoffs in performance, cost, and durability.
Chemistry doesn’t just live in labs and safety manuals. Understanding the real formula of compounds like toluene-4-sulphonohydrazide means connecting the dots from molecular structure to industrial application—all the way to health, safety, and sustainability. Small details—like the presence of a single sulfur or a second nitrogen—shape everything from air quality controls to whether shoes and toys are safe in a child’s hand.
Toluene-4-Sulphonohydrazide doesn’t show up in most people’s daily lives. As someone who spent a chunk of their early years around industrial labs, I know chemicals like this don’t make headlines, but improper storage can create serious headaches. Toluene-4-Sulphonohydrazide can break down when exposed to the wrong temperatures and conditions, and its decomposition isn’t harmless. Anyone handling this compound ought to respect it—the risks don’t respect ignorance.
In practice, storage temperature separates safe handling from costly mistakes. I’ve seen what happens when labs push limits or cut corners; even a few degrees too high can kick off unwanted reactions or lead to dangerous byproducts. Toluene-4-Sulphonohydrazide prefers cool, dry spaces. Refrigeration around 2–8°C puts minds at ease and extends shelf life. Room temperature can slide by, but heat accumulations in poorly ventilated storage areas bring trouble. Nobody wants to deal with chemical breakdowns that could spark fires or release harmful vapors.
Moisture doesn’t shout its arrival. One day the bottle looks fine. Later, you open it, and there’s clumping or signs of caking. I learned early that water sneaks in quickly—even from sweaty hands. For this reason, containers deserve special attention: always close lids tightly, use desiccators, and pick containers that fend off damp air. Silica gel packets inside cabinets help, too. It’s easy to overlook small habits, but those details keep a hazardous mess off the workbench.
Glass containers beat plastics every time for holding up against this kind of chemical. Plastic can degrade and might react, turning containment into contamination. I stick with amber bottles when possible—they add a layer of defense against light, which sometimes nudges sensitive chemicals to react. Labels need to be clear and easy to read, not just for compliance, but so anyone on the team can quickly tell what’s inside without guesswork.
Labs and workshops fill up with all kinds of reagents. It’s tempting to line them up in one go, but that’s asking for disaster. I always keep Toluene-4-Sulphonohydrazide away from oxidizers, strong acids, and sources of ignition. One shelf, one type. Accidental mixing doesn’t just waste material, it threatens lives. Last year, I heard about a near miss after incompatible powders got mixed during a rushed day—luckily there was plenty of ventilation, but cleanup lasted hours.
Years back, gloves ripped and I touched a bit of sulphonohydrazide. It got handled fast—washed off right away—but the experience stuck. Chemicals don’t ask if you’re having a good day. They require respect, attention, and proper gear. I follow every storage protocol because the alternative brings risk right to the front door. Training new team members, I emphasize real stories over regulations. People remember stories long after they forget rule numbers.
Get a dedicated storage area. Invest in good ventilation and refrigeration. Stock spill kits and make sure they’re up to date. Check container seals routinely, not once a year. Don’t let a habit of “just tuck it anywhere” take root; disciplined storage pays off in safety and efficiency. Safety audits uncover weak spots, and regular drills make sure everyone stays sharp. Much of chemical safety doesn’t feel exciting—until it’s desperately needed.
Responsible storage of Toluene-4-Sulphonohydrazide keeps people, property, and the environment out of harm’s way. Treating these basics as daily practice, instead of yearly reminders, keeps labs running smoothly and workers heading home safe.
Toluene-4-sulphonohydrazide shows up in laboratories and factories where chemical blowing agents are part of the process. People working with this powder know it brings risks that need real respect and solid procedures. Over the years, I’ve watched seasoned techs, scientists, and handlers follow strict habits, not out of compulsion, but because experience teaches that a single slip can have big consequences.
Anyone who’s spent time with this substance will tell you—bare hands and open faces don’t belong anywhere near it. Standard protocol calls for chemical-resistant gloves and tight-fitting safety goggles. Lab coats with cuffs snapped or elasticized keep powder from clinging to wrists. In settings where dust floats around, respirators become essential. The fine particles cause lung irritation, and inhalation over time can set up chronic symptoms. A simple N95 mask usually doesn’t cut it; full-face respirators fitted with appropriate cartridges stand as the real line of defense.
It only takes a few minutes for poor ventilation to turn a routine transfer into a hazard zone. Proper fume hoods or local exhaust systems, checked and serviced on a schedule, protect everyone in the room. I remember a cramped workspace with just a tired old fan pushing air, and the headaches that followed made it clear—nothing beats purpose-built air extraction. Periodic air-quality checks keep the danger in check and give workers confidence.
Spills happen, even for the most practiced hand. Quick response matters. Spilled Toluene-4-sulphonohydrazide should never get swept or vacuumed up with regular equipment—HEPA-filtered vacuums or damp disposable cloths do a better job limiting dust clouds. Workers wear their full protective gear and seal the residue in labeled, chemical-resistant bags or containers. These details make the difference between a contained problem and a contamination issue that spreads through a facility.
Direct contact leads to rashes, redness, or eye irritation. Immediate flushing with water—fifteen minutes for the eyes, several for the skin—reduces the chance of long-term damage. Emergency eyewash stations and showers don't belong boxed in a corner; they need to be within arm’s reach. I’ve seen these lifesavers overlooked in design, then replaced only after an incident wakes management up to their absence.
New workers pick up on attitudes fast. If veterans take shortcuts, newcomers do too. Proper onboarding involves hands-on demonstrations and real talk about past near-misses, not just a stack of papers to sign. Regular refreshers keep everyone’s habits sharp. Leadership can’t treat training as a box to check; it’s an ongoing investment in the team’s health and safety.
Adequate storage reduces unplanned exposure. Tightly sealed containers, marked with clear and visible labels, stay in cool, dry rooms away from acids, bases, and other reactive compounds. Keeping an eye on inventory prevents old stock from degrading and building up unnecessary risks. Responsibility for this job often rotates among staff, but having a clear point person ensures accountability.
Accidents and close calls shape the best safety routines. After each incident, take the time to run through what went wrong, share the lesson, and adjust the protocol. Document changes and make sure everyone understands the new expectations. This cycle of improvement grows a culture where people watch out for each other and keep the bar high for safety.
People working with chemicals get used to remembering long names and complicated formulas, but mistakes happen when a compound’s identity isn’t clear. Toluene-4-Sulphonohydrazide, for example, shows up in a lot of technical and manufacturing places. Asking for the CAS number of Toluene-4-Sulphonohydrazide points straight to the backbone of chemical safety: both its proper identification and traceability. For reference, the CAS number for Toluene-4-Sulphonohydrazide is 80-51-3.
Anybody who’s ever scoured an old label in a storage room, or tried hunting through stacks of old technical sheets, knows confusion takes over when names change or suppliers use different terms. The CAS number exists to keep things straight. It’s not just for the paperwork; it stops the kind of errors that have real-world consequences. Once, during a school lab project, I saw what happened when students used two chemicals with similar names. Wrong chemicals led to wasted time and a lot of worry about what had been mixed together.
Industry professionals rely on the CAS system every day. Manufacturers of foaming agents or plastics, for instance, check the CAS number 80-51-3 to make sure they’re using the right compound. This matters for everything downstream, from product safety to worker health. The CAS number strips away the uncertainty, so everyone stays on the same page, regardless of local language or naming customs.
Many chemicals have both obvious uses and hidden dangers. Toluene-4-Sulphonohydrazide, often used as a blowing agent in making foamed plastics, brings up serious health concerns if handled incorrectly. The Material Safety Data Sheet connects to the CAS number, linking anyone on the job — chemist or technician — to all vital hazard data: toxicity, safe handling, and spill instructions.
In my own experience working alongside small-scale manufacturers, I’ve seen how easy it becomes to mix up suppliers’ product codes or trade names. Using the CAS number on every order and worksheet ended up saving more trouble than anyone expected. It might seem like a small detail, but it drives home the point that safety starts well before chemicals even reach the factory floor.
Regulators look for CAS numbers because they want clean records — not just on safety, but also on sourcing and recall management. Global companies dealing with export controls, REACH registration in Europe, or EPA regulations in the US need nothing left to chance. Tracking by CAS number means recalls and compliance audits have a clear trail. This saves time and money, but more important than that, it builds trust.
A lot of harm comes from small mistakes in identification. Toluene-4-Sulphonohydrazide with CAS number 80-51-3 needs to stay unique in every database and on every safety sheet. Using plain, exact global codes forms the core of building a culture of accuracy and responsibility in both labs and factories.
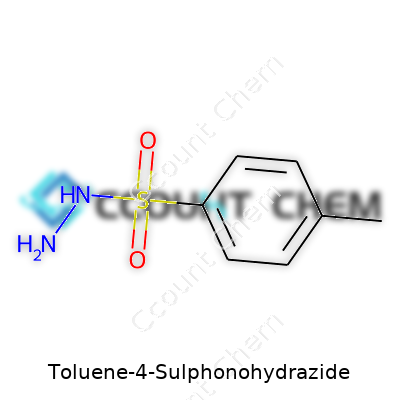
| Names | |
| Preferred IUPAC name | 4-methylbenzenesulfonohydrazide |
| Other names |
P-Toluenesulfonylhydrazide 4-Methylbenzenesulfonylhydrazide TSH |
| Pronunciation | /ˈtɒljuː.iːn fɔːr sʌlˈfəʊnəʊ.haɪˈdreɪzaɪd/ |
| Identifiers | |
| CAS Number | 80-51-3 |
| Beilstein Reference | 136979 |
| ChEBI | CHEBI:87329 |
| ChEMBL | CHEMBL3209601 |
| ChemSpider | 22431 |
| DrugBank | DB14096 |
| ECHA InfoCard | 100.019.380 |
| EC Number | 200-772-2 |
| Gmelin Reference | 82148 |
| KEGG | C14317 |
| MeSH | D017239 |
| PubChem CID | 158888 |
| RTECS number | WO5950000 |
| UNII | 1LCS1526SO |
| UN number | UN2468 |
| Properties | |
| Chemical formula | C7H10N2O2S |
| Molar mass | 186.22 g/mol |
| Appearance | White to yellowish crystalline powder |
| Odor | Odorless |
| Density | 1.33 g/cm³ |
| Solubility in water | Insoluble |
| log P | 0.01 |
| Vapor pressure | 0.000166 hPa (25°C) |
| Acidity (pKa) | 8.94 |
| Basicity (pKb) | 11.0 |
| Magnetic susceptibility (χ) | -48.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.587 |
| Dipole moment | 3.50 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 321.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -59.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6607.0 kJ/mol |
| Pharmacology | |
| ATC code | N01AX10 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H332: Harmful if swallowed or inhaled. |
| Precautionary statements | P210, P261, P280, P301+P312, P304+P340, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Flash point | 128°C |
| Autoignition temperature | 400°C |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 2400 mg/kg |
| NIOSH | SW4380000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Toluene-4-Sulphonohydrazide: Not established |
| REL (Recommended) | 0.6 mg/m3 |
| Related compounds | |
| Related compounds |
Benzenesulfonyl hydrazide p-Toluenesulfonyl chloride p-Toluenesulfonamide p-Toluenesulfonic acid Sulfanilhydrazide 4-Nitrobenzenesulfonyl hydrazide |