In the world of ionic liquids and novel fluorinated compounds, Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate stands out as a result of decades of exploration in fluorine chemistry and specialized salt synthesis. Industrial chemists seeking safer and more stable alternatives to traditional solvents found that incorporating robust alkylphosphonium cations and perfluoroalkyl sulfonate anions could result in a family of salts that are both thermally stable and chemically resilient. The drive to displace legacy halogenated solvents, once prized for their performance but found wanting in terms of health and environmental safety, spurred research that led to the development of these ionic liquids through collaborations between academic researchers and multinational specialty chemical producers, especially starting in the late 1990s and early 2000s, riding a global wave of green chemistry initiatives.
Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate exists as a salt that can act either as an ionic liquid or as a functional additive in a host of advanced material systems. Each molecule features a large, hydrophobic phosphonium cation paired with a heavily fluorinated, robust sulfonate anion. This unique structure imparts several practical properties that attract both industrial users and research laboratories. You find this compound less often on a general catalog or commodity list, but more as a customized material in surface engineering, electrochemistry, or advanced synthetic protocols, appreciated for its unusual compatibility in systems that demand both thermal stability and very low reactivity with water or organic reagents.
An unmistakable odor and an oily liquid or waxy solid appearance often characterize these compounds, with physical form shifting based on ambient temperature and purity. Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate delivers a melting point well above room temperature for its ionic variants or sits as a viscous fluid among certain product grades. The perfluorinated sulfonate group gives extraordinary chemical resistance, leaving this salt unaffected by strong acids, bases, and even oxidizing agents that corrode ordinary organic compounds. Conductivity, a critical point for its popularity in electrochemical cells, rivals that of more traditional imidazolium or pyrrolidinium-based salts, but the phosphonium core often resists decomposition under intense electrolysis, opening up paths to longer equipment lifespans.
Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate typically arrives as a solid or viscous liquid, delivered in sealed glass or chemical-resistant plastic bottles. Key specifications revolve around purity, moisture content, and the proportion of butyl chain branching or impurity cations. Standard information includes storage temperature, light and air sensitivity, and conformance to specific analytical tests—like NMR or ion chromatography—to confirm absence of chloride, nitrate, or heavy metal traces. Labels provide batch numbers, recommended shelf life, and emergency contact details, because practical handling in research or manufacturing demands complete traceability and up-to-date safety knowledge at every stage of use.
The main synthesis route builds from the reaction of tetrabutylphosphonium halide—often the chloride or bromide—with nonafluorobutane-1-sulfonate potassium or sodium salt through direct aqueous or biphasic salt metathesis. Careful control of recrystallization and phase separation steps yields a high-purity product, with any halide or alkali metal residues removed using multiple washes with ultrapure water or organic solvents such as acetonitrile or dichloromethane. Every step requires analytical verification because even trace impurities can alter the performance of this material in batteries, catalysts, or coatings, especially at the scale required for electronics manufacturing or energy storage research.
This phosphonium salt remains chemically rugged, rarely reacting with strong acids, bases, peroxides, or oxidizers. That chemical toughness makes it a go-to agent in demanding settings like lithium battery electrolytes, where lesser salts break down. On rare occasions, the butyl chains may undergo slow oxidation under intense heat or in the presence of radical initiators, and strong nucleophiles might attack the phosphonium core, an effect more common with smaller alkyl substituents. Modifications focus on the selective exchange of the sulfonate group with various perfluoroalkyl tails to tune hydrophobicity or ionic conductivity according to the needs of a specific process or device.
Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate is often abbreviated as [Bu4P][NFBS] across academic and commercial literature. It also turns up under names like Tetrabutylphosphonium perfluorobutanesulfonate, TBP-NFBS, or perfluorobutyl sulfonate phosphonium salt, reflecting differences in supplier or national regulatory guidelines. Researchers may encounter minor spelling variations when digging through material safety data sheets or research papers, but the key identifiers always focus on the tetrabutylphosphonium cation linked with the nonafluorobutane sulfonate anion.
Personal experience in chemical handling puts safety squarely at the forefront. Although Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate resists thermal decomposition up past 200 °C, it deserves full respect. Direct skin contact or inhalation of dust or aerosols requires gloves, goggles, and handling in ventilated hoods—phosphonium salts can irritate skin and mucosa, and no material containing perfluoroalkyl groups should be assumed benign without data. Facility managers mandate secondary containment, waste disposal through certified hazardous waste contractors, and regular review of safety protocols aligned with OSHA and REACH guidelines. Emergency information must stay visible because risks shift during scale-up or long-term storage, raising the stakes for industrial users.
This salt’s rich constellation of attributes draws attention from battery specialists, catalysis researchers, and advanced coating developers. In lithium-ion or sodium-ion battery research, [Bu4P][NFBS] shows high oxidative stability and compatible viscosity at elevated temperatures—qualities crucial for next-generation electrolytes that demand more than the typical organic carbonates can supply. Fluorinated sulfonate anions deliver low flammability and create solid interfaces on metal electrodes, pushing the envelope for fast-charging and long-life cycles. I’ve also seen this compound evaluated as a phase-transfer catalyst in selective alkylation and cross-coupling reactions, where its ionic liquid character accelerates slow reactions and opens up the possibility of new routes to complex molecules. In antistatic and hydrophobic surface treatments, the presence of the perfluorobutyl group gives water and oil repellence while providing electrical conductivity where more traditional surfactants fail. Electronics fabricators and specialty coating formulators look to this material for new ways to protect sensitive hardware from humidity, corrosion, or dust.
Teams worldwide keep pushing Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate into new territory. Lab notebooks grow crowded with work on custom electrolyte blends for solid-state batteries or supercapacitors. Engineers treat the balance between conductivity and viscosity as the key challenge—one that requires precise adjustment of cation size, anion fluorination, and salt concentration. Synthesizing derivatives by changing either the phosphonium substituents or the fluoroalkyl sulfonate domain lets teams dial in the right mix of conductivity, toxicity, and environmental stability for each use case. Companies sometimes fund internal toxicology and biodegradation studies to stay ahead of evolving regulations and keep intellectual property firmly in their hands.
Anyone using a heavily fluorinated ion knows the scrutiny from health and environmental regulators runs high. Toxicity assessments, both acute and long-term, drive careful study of Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate. Researchers test effects on aquatic life and bioaccumulation under conditions ranging from environmental spill exposure to intentional incineration. Early work points to less acute toxicity versus legacy perfluorooctane sulfonate (PFOS)—a chemical now largely banned—but cautious voices call for expanded studies on breakdown products and their environmental fates. Workers in the field comply with exposure limits, regular bloodwork, and advanced environmental monitoring practices, following best practices until universal safety consensus emerges.
Looking forward, Tetrabutyl-Phosphonium Nonafluoro-Butane-1-Sulfonate stands poised to take center stage in several technology shifts—energy storage, electronics miniaturization, and green manufacturing all need better, safer, more rugged materials. Demand for new battery electrolytes, antistatic coatings, and stable surfactants keeps mounting, driven by electrification and digitalization on every continent. Though regulation on perfluorinated compounds gets stricter each year, ongoing work on safer degradation, closed-loop recycling, and low-toxicity modifications points toward a future where these advanced salts support key industries without repeating past mistakes about environmental and occupational harm. Chemists, engineers, and manufacturers share both the responsibility and the excitement as they build on the strong foundation laid by the last two decades of research and product development.
I’ve spent my share of hours scanning through product lists in chemical catalogs, and every so often, a new ionic liquid pops up, loaded with syllables, that promises to do what previous materials couldn’t. Tetrabutyl-phosphonium nonafluoro-butane-1-sulfonate fits that bill. This compound turns heads in labs thanks to its stable and highly tunable nature. I remember the first time our group handled these “designer salts,” and realized they didn’t just replace older solvents—they started opening doors in clean tech and advanced manufacturing.
Let’s be clear. Fluorinated compounds often catch bad press because of environmental persistence, yet they offer unrivaled chemical performance. This salt carries a bulky nonafluoro-butane-1-sulfonate anion. The phosphonium cation, in turn, packs four butyl groups. That hybrid brings together strong chemical stability, low flammability, and an ability to stay liquid over a vast temperature range.
In electrochemical cells, researchers hunt for stable yet conductive liquids, and this phosphonium salt emerges as a top contender. Traditional electrolytes based on organic solvents spark safety headaches—volatile, sometimes toxic, always finicky. Tetrabutyl-phosphonium nonafluoro-butane-1-sulfonate doesn’t carry those burdens. It survives high voltages, supports smooth ion flows, and resists breakdown. I’ve seen it listed for redox flow batteries and next-gen lithium cells, where safety and long cycle life often matter more than cost.
As a solvent, it supports difficult catalytic transformations. Chemists facing stubborn bonds, especially ones with fluorinated partners, have started using ionic liquids like this to coax out better yields from tough reactions. The strong, yet inert, environment seems to help with fluoro-organic coupling and can ease recovery since products typically separate cleanly from the ionic phase.
Fluorinated chemicals need careful handling and clear end-of-life planning. People who work in academic and industry settings ask tough questions about persistence. I’ve seen growing calls for proper recycling and containment, instead of older routines that assumed waste streams take care of themselves. Regulatory approaches remain in flux, but safety data are improving. Material safety sheets flag low vapor pressure and resistance to combustion, both plus points in labs worried about fire or inhalation.
No one expects a single compound to solve every battery, catalysis, or solvent challenge. Still, the phosphonium class keeps inching forward in scaleup. Producers have started reducing environmental impact by scaling more responsibly and taking feedback from downstream users about what matters: purity, reliable supply, and clear communication on toxicity.
Some engineers look for ways to recover and reuse these liquids. Technologies for ionic liquid purification continue to improve, so the costly task of making a high-grade product doesn't turn into a sinkhole. More collaboration between manufacturers, users, and regulators helps avoid regulatory whiplash and ensures these compounds actually deliver their promised benefits.
As chemical industries lean into electrification, cleaner synthesis, and longer-lived materials, compounds like tetrabutyl-phosphonium nonafluoro-butane-1-sulfonate will keep popping up on order sheets. Whether in a flow battery pilot project, a green chemistry startup, or a research lab, adoption rises or falls on real safety data and results at the bench.
Years of working near harsh lab materials drive home the lesson: chemicals don’t care if you’ve had a rough day or if you’re hurrying to finish a job by five. Skipping basic precautions can lead to skin burns, lung problems, or even hospital visits. Even common lab solvents or cleaning acids quietly go from harmless to hazardous in an instant. Respect for the hazards starts when you admit mistakes come easy in distracted moments.
Protective gear meets the threat at the front door. Gloves, goggles, and sturdy lab coats go a lot further than just ticking a box on a safety checklist. A cheap pair of gloves keeps strong acids off your hands, while splash goggles mean an accident stays inconvenient, not scarring. Once I watched a splash that missed an unprotected colleague’s eyes by an inch; it’s hard to forget the relief on their face. OSHA—America’s top workplace safety agency—records nearly seventy thousand chemical injuries in labs each year, with most involving the eyes or skin. That tells me the basics matter.
Half the trouble starts when people cut corners reading labels, sometimes trusting faded handwriting or sketchy old containers. Clear, up-to-date chemical labels—and unopened safety data sheets—mean everyone knows what’s inside and what can go wrong. I once mixed two nearly identical bottles on a cluttered shelf. Thanks to a quick double-check, disaster never materialized. Properly labeled supplies prevent “lookalike” mistakes that could cost a limb, not just a lunch break.
Fume hoods don’t just keep air fresher—they block out poisons before anyone realizes something’s wrong. Many solvents and powders evaporate and fill a room while workers barely sense a smell. One time, a colleague decided to clean glassware using acetone outside the hood after hours. The sharp scent lingered for hours and headaches followed. This confirmed that hoods aren't just expensive furniture. Labs and workshops should always draw fumes away, and fans or hoods require regular checks for blockages or leaks. The science on this is clear: proper ventilation drops illness rates and prevents disaster.
Spills turn up when folks think they’re done with the hard part. Fast, careful cleanup—using the correct neutralizers or absorbent materials—makes forgotten puddles and powder residues less likely to haunt others later. My clean-up routine includes a final wipe-down and a check under workbenches, since risky dust loves to gather where no one looks. It’s not just about the next shift but the memory of small spills that led to big problems a little further down the line.
Nobody handles chemicals alone. Good safety habits grow from group reminders and regular training, not from reading one booklet on a first day. Healthcare and manufacturing industries that refresh chemical safety skills every few months see lower accidents, according to the CDC. I share stories with new team members about close calls, so those lessons last beyond orientation.
People learn shortcuts by watching others. Sharing quick, honest stories about times when safety steps made a difference reinforces why rules matter. I always set out extra gloves and post signs about last year’s close call, not to scold, but so the reminder feels real. Building strong safety culture lives in those everyday choices. The point of every step—whether that’s double-checking a cap, labeling leftovers, or never skipping goggles—is not just about ticking boxes but protecting real lives, including our own.
Science often gets painted as mysterious, guarded behind layers of jargon and lab coats, but at the end of the day, molecules build everything we touch. The chemical structure and molecular formula of a compound are much more than academic details. They show us why a compound acts the way it does, what it can latch onto, and where it fits in the bigger picture—medicine, industry, or even the food on your table.
Years ago, I remember seeing a skeletal formula drawn on a classroom chalkboard—lines meeting at angles, some labeled, some not. It looked simple. And it clicked for me: that drawing was the shortcut to understanding reactivity, safety, even how that molecule would move through water or fat. The structure, often shown in a two-dimensional form, packs in all those details—how atoms connect, what types of bonds lock them in place, and which groups stand out and react. Noticing an oxygen glued to a ring? That can tell you about polarity, and even flag up a possible risk if that compound ends up in your bloodstream.
Pick up a bottle with a label that lists C8H10N4O2 and you’re holding the same stuff that gives your coffee a jolt — caffeine — neatly summed up in a few letters and numbers. That formula spells out the number and kind of each atom. It’s a code, but one that translates into real-world impacts. Short on oxygen or stacked with hydrogens? It can mean the difference between a painless painkiller and a dangerous narcotic.
In recent years, families have heard about recalls—contaminated food, mislabeled medications. The ability to pin down molecular structure stops those problems before they start. Researchers and regulators use this knowledge to spot adulterants, contaminants, or to keep out counterfeits that look right on a shelf but fall apart under a microscope. If you can’t draw the molecule, you’re trusting blind.
Accurate formulas and clear structures back up trust. They allow pharmacists to swap a branded drug for a generic, or let brewers tweak a yeast strain for a new IPA. In agriculture, they help create fertilizers that drop nutrients exactly where roots need them, not washing away into rivers and compounding drought. My neighbor once switched fertilizers based on a friend’s advice, burned half his tomato plants, and wondered where he’d gone wrong—the label didn’t spell out what was inside.
For anyone working in health, food, or engineering, knowing the structure means new ideas don’t start from scratch. Chemists build on what’s proven. Molecular diagrams guide improvements in battery technology, plastics that actually biodegrade, or cleaner fuels. Sharing clear structural information speeds up progress. If every manufacturer opened up about the real compounds inside, mistakes and waste would shrink.
Right now, most consumers skim over formulas and structures—either not caring or feeling overwhelmed. Breaking down these codes through simple education in schools, product guides, or even supermarket signage can close the gap. We all deserve to know what we’re buying, eating, and applying. The basics of structure and formula are as much about empowerment as they are about chemistry. Relying on clear, accurate science helps everyone make informed choices, whether you’re testing groundwater or brewing your morning cup.
Tetrabutyl-phosphonium nonafluoro-butane-1-sulfonate stands out as a strong player among ionic liquids. With the swelling interest in greener chemistry and the push for more precise catalysis, this salt finds its way onto more shelves. I’ve seen labs light up with new synthetic possibilities, but excitement quickly sours when safety gets ignored. No shortcut ever saved a chemist from the long-term mess of improper storage.
This kind of compound—at least in labs I’ve worked—gets temperamental in the face of moisture and heat. Left next to a window on a summer day, the structure starts breaking down too fast, and you end up losing both your money and your reliability. Every time I open a poorly sealed jar, I spot the clumps and can almost smell the wasted grant funding.
Dry, cool storage becomes non-negotiable. Common sense goes a long way: use a desiccator or inert gas cabinet, and never store anything like this above room temperature. I’ve seen glassware fog up in humid storerooms. Picture all that water vapor sneaking its way into your chemical and kicking off unplanned reactions. Most quality control failures in big batches come straight from ignoring these risks. That tells me this isn't a problem for the future; this is negligence happening now.
Direct contact with these ionic liquid salts can irritate the skin, and breathing in dust from clumsy transfers hurts. My lab coat remembers the first time I didn’t wear gloves: a red patch that stung for days. Fume hoods, eye protection, and gloves are not overkill in this setting; they count as baseline respect. I ask new students: would you eat your lunch next to this bottle? The answer’s always no—so why would you leave it by an open sink, or let the lid sit loose?
Labeling makes a difference, too. More than once I’ve watched people guess at a white powder in a flask, only to realize too late they’ve been handling something more reactive. Even if you think you’ll remember the code or color, clear, specific bottle labels keep everyone in the loop. Write the date opened and the initials of the handler, so stewardship becomes something you can trace.
Long-term storage works best if people don’t go rogue. Keep a shared log of who’s checked on the material and when. This isn’t bureaucracy; it’s the adult version of checking if you locked the back door—basic practice that saves the insurance headaches down the line. We keep silica gel packets in the jars and swap them every month without fail. More than once, we’ve caught issues early just by keeping good notes.
Some ionic liquids build up static or degrade under light. Store bottles in opaque secondary containers if you share fridge space with photosensitive material. Accidents don’t just happen because of big spills; a slow leak from a cracked lid into shared storage leads to a lost weekend and tainted samples.
Disposal plans should stay up to date. Outdated salts turn hazardous if they wander off to the general waste bin without steps for neutralization or proper containment. Forgetting old stock at the back of a drawer is a gamble I’ve seen smart folks regret. Everyone gains by keeping a rolling inventory and treating old chemicals as candidates for end-of-life planning.
Good storage isn’t solved with just shiny new cabinets or expensive gasses. It starts with a culture where everyone watches out for each other and respects the science enough to avoid shortcuts. Building trust in your processes keeps people safe, cuts costs, and lets the real work—discovery and progress—unfold without unnecessary risk.
Anyone who’s spent time on a shop floor or worked in a lab knows the headache that comes with mixing unfamiliar chemicals. It’s not just about whether something dissolves or blends. Sometimes, even a harmless-looking liquid can cause foam to boil over, corrode equipment, plug a filter, or worse, trigger a hazardous reaction. Most folks forget that each chemical has its own quirks. Overlooking those can halt a whole process or endanger crew safety. That sort of real-world knowledge beats anything you’ll get just from glancing at a label.
Safety Data Sheets and technical documents usually give a rundown on basic incompatibilities—don’t mix bleach and ammonia, keep acids away from cyanides. Yet there’s more buried in the details. For example, if a paint thinner works fine with water or mineral spirits, that doesn’t guarantee it’ll mix safely with every anti-rust additive or cleaning solvent on a shelf. Sometimes two products slide together like oil and water, sometimes you get sludge, and sometimes you get heat, fumes, or a wrecked pump.
In my own experience, new team members make the mistake of trusting experience from one job and expecting the same results elsewhere. In one case, a commonly used solvent for cleaning turned rubber gaskets in a transfer pump gummy and useless in a matter of hours. Replacing one gasket is nothing, but if it shuts down a whole line, you’ve got a bigger problem.
Regulatory and technical authorities like OSHA and the National Fire Protection Association publish clear-cut lists about what chemicals shouldn’t be mixed. The American Chemical Society pulls no punches on lab accidents related to poor assumptions around compatibility. A 2016 industry report from ChemSec showed that over 20% of process incidents resulted from untested combinations, not just operator error or equipment malfunction. These days, most companies also run smaller-scale tests before introducing a new product into larger systems.
Manufacturers sometimes publish compatibility charts, and these help. Still, those resources have limits. Nobody tracks every possible combination, especially for newer specialty formulations. In fast-paced setups—like contract manufacturing, R&D, or even janitorial work in schools—workers blend chemicals based on urgency, not always based on data.
Relying only on paperwork or instincts never cuts it if you want a safe, smooth operation. Teams that see the fewest mishaps keep their own written logs about reactions, not just hazard scores. They take small bench-top samples, use glass and metal containers for initial tests, and jot down notes about anything strange—color shifts, cloudiness, heat. One veteran of the coatings industry I worked with kept a beat-up binder full of hand-written tables, stories about past failures, and warnings to future users.
Digital tools help reduce mistakes. Inventory management systems with compatibility checkers cross-reference ingredients, and set off alarms if anything looks sketchy. Still, there’s no substitute for real training, honest feedback from shop veterans, and the good habit of reading both the label and the fine print. New operators should ask direct questions and document every surprise, not just what worked.
Mixing chemicals isn’t just about following instructions. It’s about understanding each product’s personality, respecting differences between batches, learning from mistakes, and sharing that knowledge. Simple test runs, frequent communication, and open records turn compatibility from a guessing game into a routine skill.
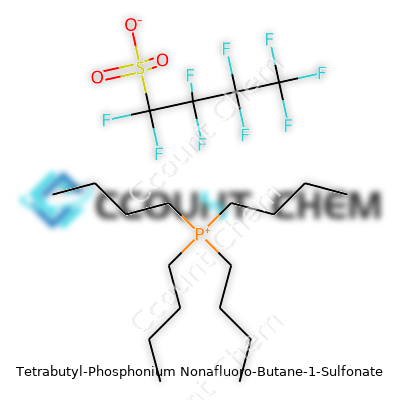
| Names | |
| Preferred IUPAC name | tetrabutyl(phosphonium) 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate |
| Other names |
Tetrabutylphosphonium nonafluorobutanesulfonate TBPFB Nonafluorobutanesulfonic acid tetrabutylphosphonium salt |
| Pronunciation | /ˌtɛtrəˌbjuːˈtaɪl fɒsˈfəʊniəm ˌnɒnəˌflʊəroʊ bjuːˈteɪn wʌn sʌlˈfəʊneɪt/ |
| Identifiers | |
| CAS Number | 344789-06-0 |
| 3D model (JSmol) | `/showmol.cgi?sid=8958bfa115eeb68b&mol=Tetrabutyl-Phosphonium_Nonafluoro-Butane-1-Sulfonate&bgcolor=0xffffff&width=400&height=400` |
| Beilstein Reference | Beilstein Reference: 3977782 |
| ChEBI | CHEBI:136844 |
| ChEMBL | CHEMBL4294742 |
| ChemSpider | 25414555 |
| DrugBank | DB11106 |
| ECHA InfoCard | 13e57352-588c-4395-b4cd-2ad7ea73e876 |
| EC Number | 429-270-0 |
| Gmelin Reference | 108618D |
| KEGG | C18773 |
| MeSH | D000073590 |
| PubChem CID | 102127312 |
| RTECS number | BO2007000 |
| UNII | 1B2Q2Q7N8Y |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID90865242 |
| Properties | |
| Chemical formula | C16H36F9O3PS |
| Molar mass | 526.50 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.16 g/cm³ |
| Solubility in water | soluble |
| log P | -0.68 |
| Vapor pressure | 0.00001 mmHg @ 25 °C |
| Acidity (pKa) | -3.8 |
| Basicity (pKb) | 13.3 |
| Refractive index (nD) | 1.390 |
| Viscosity | 176 mPas (25°C) |
| Dipole moment | 3.4943 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 810.6 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1473.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0-Yes |
| Flash point | > 113 °C |
| Lethal dose or concentration | LD50 Oral Rat > 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| REL (Recommended) | 38.2 mg/L |
| IDLH (Immediate danger) | NIOSH: Not established |
| Related compounds | |
| Related compounds |
Tetrabutylphosphonium bromide Tetrabutylphosphonium chloride Tetrabutylphosphonium hydroxide Tetrabutylphosphonium fluoride Tetrabutylphosphonium methylsulfate Tetrabutylphosphonium bis(trifluoromethylsulfonyl)imide |