Tert-Butyldimethylsilyl trifluoromethanesulphonate, often called TBSOTf for short, draws its roots from the boom in organosilicon chemistry that took off in the mid-20th century. Before researchers started relying on TBS-ether groups for protection, chemists found themselves grappling with reactions that overreached, went off course, or just plain failed. The demand for a more reliable method to protect alcohols during multistep syntheses led innovators to the drawing board. Silicon-based protecting groups answered the call, especially after the limitations of classical solutions like benzyl or methyl ether groups became apparent. From experience working in synthetic labs, you see firsthand how older approaches required harsh conditions, often leading to collapse of sensitive molecules. TBSOTf, as a reagent, began cementing its value among organic chemists in the late 1970s and 1980s. Since then, its reputation as an efficient silylation agent has only grown.
TBSOTf makes life easier for chemists tackling complex routes. Its primary role centers on transforming alcohol functional groups into their tert-butyldimethylsilyl (TBS) versions, locking them away from unwanted side reactions. This transformation proves especially useful in medicinal chemistry and total synthesis where you spend weeks building up a molecule, only to risk losing half your work because of one exposed alcohol group. TBSOTf earns praise for working under milder conditions than its silyl chloride cousin, sidestepping strong bases that can scramble delicate reactants. Its broad utility comes from that gentle touch—less hassle during introduction, again when you eventually need to pull off the TBS group.
TBSOTf comes as a clear to yellowish liquid, often described as volatile and moisture-sensitive. It gives off a noticeable odor. Anyone who's handled triflate reagents recognizes that scent in a heartbeat. Its molecular formula reads C8H18F3O3SSi, with a molecular mass just shy of 314.4 g/mol. TBSOTf reacts swiftly with water—even ambient humidity will hydrolyze it if care slips for a moment. In dry systems, the reagent remains stable, but the moment a wet glove or vessel enters the mix, breakdown isn't far behind. Its density stands around 1.2 g/cm³, and it boils at roughly 57°C (at 10 mmHg), demanding tight control both in storage and use.
Bottles of TBSOTf arrive capped tight with PTFE liners, often wrapped under an inert atmosphere. Most suppliers distinguish high-purity lots for demanding applications—99% or better—listing trace impurity profiles right on the certificate of analysis. You won’t spot it on a hardware-store shelf. Chemical labeling features non-reactive glass jugs, stoppers sealed to keep out atmospheric moisture, with hazard pictograms for corrosivity and acute toxicity according to GHS standards. In workspaces following responsible practices, every bottle lands in secondary containment, with inventory sheets updating lot numbers and received dates to track shelf life and batch variations.
Industrial prep for TBSOTf calls for dry, oxygen-free process streams. Starting from tert-butyldimethylsilyl chloride and silver triflate, the reaction proceeds in a non-protic solvent like dichloromethane. Silver chloride precipitates, marking conversion. In the lab, I've watched technicians work behind shields, loading silver salts slowly to avoid clumping or local hot spots. Post-reaction, filtration removes insoluble byproducts, and rotary evaporation strips off volatile organics. Some commercial syntheses scale this up, but every step stays dry. Any slip leads to costly batch failures thanks to the sensitivity of TBSOTf to water.
TBSOTf serves up a powerful silylation punch. Alcohols and some amines snap up the TBS group even at low temperature, with mild bases like 2,6-lutidine often smoothing out the process. Try running the same experiment with TBS chloride—reaction times stretch long, and yields shrink unless stronger conditions force the reaction. I've seen firsthand how TBSOTf upgrades the route, especially for substrates with acid-labile or base-sensitive motifs. Beyond simple protection, TBSOTf sometimes finds use in activating nucleophiles or as a dehydrating agent, though these uses see less routine attention. Key reaction partners include everything from simple sugars to tricky peptides. Deprotection—getting rid of the TBS—usually employs fluoride sources or strong acids, and the clean break helps chemists recover products in high purity.
TBSOTf goes by several trade and systematic names. Chemists sometimes refer to it as tert-butyldimethylsilyl triflate. The IUPAC mouthful, tert-butyldimethylsilyl trifluoromethanesulfonate, rarely shows up outside paperwork. On procurement forms, you find CAS number 69739-34-0, which spares confusion from ambiguous short names. Some companies use shorthand like TBDMSOTf, reflecting the same chemical with a twist in the abbreviation. Keeping an eye on synonyms matters, as differing catalogs from European, American, or Asian suppliers often list the reagent under one name but not another.
Experience in synthetic labs teaches deep respect for TBSOTf’s hazards. Exposure risks include severe burns from skin or eye contact, and inhalation of vapors causes respiratory irritation. Nitrile and latex gloves hold up only so long—double gloving under a fume hood becomes nonnegotiable. Spills on benchtops react with the damp air, generating hydrofluoric acid in trace amounts, which can slip past detection until it’s too late. Proper eyewash and calcium gluconate gel always stay on standby. Chemists I know recount surprise reactions between TBSOTf and accidental contaminants—even traces of protic solvents trigger vigorous, exothermic decompositions. Regular refresher safety training and written SOPs reduce incidents, but constant vigilance proves most effective.
You find TBSOTf humming in the background of academic and pharmaceutical labs worldwide. Its mainstay role is protection of alcohols and sometimes amines during complex molecule assembly, such as in the synthesis of nucleoside analogs, steroid frameworks, or in oligosaccharide chemistry. The reagent shines where substrates buckle under harsher alternatives. Medicinal chemists lean on TBSOTf when piecing together sensitive building blocks under gentle conditions. At scale, fine chemical manufacturers benefit from the clean reaction profile and fewer side products, reducing purification headaches. Contract research outfits keep TBSOTf close because it “just works” when other silylation methods stall out.
In research circles, work continues to expand the playbook of protective group strategies using TBSOTf. Projects now focus on tuning selectivity—trying to outmaneuver problems of over-silylation or cross-reactivity in complex, multi-functional substrates. High-throughput platforms allow chemists to screen dozens of reaction combinations, tracking which partners and solvents give clean, robust conversions. Recent journal reports detail clever ways to deploy TBSOTf alongside other catalysts, squeezing even more efficiency from multistep assembly lines. These advancements build confidence for both industry and bench chemists, who continuously push for shorter, greener, and higher-yielding synthetic routes.
Research shows TBSOTf’s toxicity profile demands respect, but knowledge gaps persist. Acute studies in rodents point to corrosive effects and rapid breakdown in biological settings, driven largely by its reactive triflate group and hydrolysis products. Chronic exposure remains under studied, largely due to limited workplace exposure under good chemical hygiene. Reports of skin and eye injury in the literature serve as reminders for rigorous PPE use. Environmental questions also get attention, since breakdown releases persistent fluorinated byproducts and siloxanes. Labs with green chemistry initiatives monitor disposal closely, often capturing contaminated waste streams for specialized treatment rather than routine neutralization.
Plenty of opportunities stand ahead for TBSOTf. In the near term, ongoing trends in automation and continuous-flow chemistry push suppliers to rethink how this reagent gets deployed safely at scale. Cleaner production techniques, improved packaging, and in-line monitoring make industrial usage less daunting. Green chemistry researchers test next-gen silylation agents to reduce reliance on perfluorinated starting materials, though TBSOTf remains the benchmark. Innovations in recovery and recycling of spent triflate reagents could lessen the overall environmental load. Synthetic chemists still look to TBSOTf for inspiration as they search for even more selective and user-friendly protection techniques. As molecular targets grow bigger and bolder, tools that combine power with finesse—like TBSOTf—become even harder to replace.
Every chemist working with organic molecules knows that keeping some groups safe during a reaction takes a fair bit of creativity. Tert-Butyldimethylsilyl trifluoromethanesulphonate, known in the lab as TBSOTf, fits the bill when a bulky silyl protecting group is needed. I’ve wrangled with plenty of molecules that demanded a way to mask their hydroxyl groups—otherwise, unwanted reactions can wreck a week’s work. TBSOTf makes this masking process snappy, sharper than many of the more common silylating reagents out there.
In my own experience purifying sugars and peptides, I ran into countless headaches with standard silyl protecting agents like TBSCl. They chug along OK with strong bases, but fickle substrates choke. TBSOTf brings serious efficiency because it’s far more reactive, laying down a protective TBS shield on alcohols or amines without a lot of fuss or byproducts. For people in organic synthesis, this shortcut means cleaner finishes, smoother downstream steps, and fewer chromatographic headaches when workups finally get done.
One of the more practical truths in the lab: solvent selection and temperature control leave little room for error. TBSOTf, compared to the old standards, demands less brute force—reactions run at lower temperatures and in milder solvents. Peptides I handled were always susceptible to rough conditions, so TBSOTf met that need with less collateral damage. Using TBSOTf helps lower the risk of decomposition or racemization in sensitive structures.
This ease of use isn’t just a convenience. Faster, cleaner reactions mean less chemical waste, which anyone working with hazardous solvents or limited resources will appreciate. As lab regulations tighten and costs climb, cleaner chemistry steps up as a real financial and environmental win.
People outside chemistry often miss how much safety and efficiency affect process development. Researchers constantly look for less hazardous alternatives to older reagents, some of which leave behind carcinogenic or persistent impurities. TBSOTf’s volatility and reactivity could draw concerns, but in my work, its benefits usually outweigh storage and handling challenges. With gloves, fume hoods, and a clear sense of caution, my teams have swapped out nastier reagents (think tin or heavy-metal catalysts) for TBSOTf and similar modifiers—raising lab safety without compromising progress.
Universities and scaled-up pharma labs push for green chemistry, and TBSOTf appears often in the journals, especially for its part in streamlining steps to complex molecules. The pharmaceutical world relies on protecting groups like TBS for drugs, vitamins, and natural products that degrade with standard methods. The demand rises for reagents that work more efficiently, create fewer byproducts, and lead to more reproducible manufacturing.
Handling TBSOTf calls for care—its triflate group transforms it into a potent electrophile, so skin and air sensitivity become real concerns. Having worked morning to night with such tricky reagents, I know firsthand that safer, smarter handling—glove boxes, pumps, and proper labeling—keeps labs running smoothly. Looking forward, chemists push for alternatives that offer TBSOTf’s punch but generate less hazardous waste or toxic byproducts.
Some newer silylating reagents, built from lessons learned using TBSOTf, offer greater selectivity, stability, and easier disposal. Researchers keep exploring these options, but few match the flexibility and speed TBSOTf brings for protecting vital functional groups. As more labs aim for safer workflow and smaller environmental footprints, TBSOTf and its rivals provide a solid springboard for more sustainable solutions in organic synthesis.
Tert-Butyldimethylsilyl trifluoromethanesulphonate isn’t a word that rolls off the tongue, and it doesn’t show mercy for mistakes. As someone who spent years dodging splashes and inhaling odd fumes in the lab, the respect for this reagent goes deep. It’s known for making chemical reactions work like magic, but even small spills can cause big headaches.
Friends in the synthetic chemistry field nod along when I say that proper storage is half the battle. Moisture is its enemy. Even a whiff of humidity can make it degrade. The bottle can turn into a powder keg. If you’ve ever watched someone open a poorly stored container, you’ve seen chemistry in panic mode. No one enjoys the hiss or the smell of decomposing sulphonates, and safety showers aren’t comedic relief.
Science isn’t glamorous and neither is sweating the details over climate. Storing this chemical inside a tightly sealed, chemically resistant container—think amber glass with PTFE-lined caps—keeps it stable. Tossing the bottle into a desiccator with fresh silica gel isn’t overkill. That orange-to-green transition tells you moisture is creeping in.
We use fridges with dry, cool air (around 0–4°C) for anything that can evaporate, react, or catch fire. This isn’t about paranoia. At ambient temperatures, trifluoromethanesulphonate turns more aggressive and breakdown speeds up. I’ve watched careless storage cost labs thousands of dollars and hours of clean-up.
Many labs have stories of ruined reagents. Sometimes, it’s the new student’s forgetfulness: an unlabeled, ring-stained bottle sitting beneath the sink or left out overnight by accident. Chemical inventories get tangled up. Nothing ruins a workday like noticing your precious reagent’s bottle is crusted around the cap.
Sunlight and open air aren’t just bad for people, they break down this sulphonate, making it worse for the next person who touches the bottle. My former supervisor drilled this lesson in hard. We’d label each container with the date it was opened and kept it stored in the chemical fridge behind layers of glass and plastic wrap. No shortcuts, no creative storage solutions. Labs that get too clever about “saving space” often lose more in ruined chemicals and compromised results.
Maybe you don’t think about dehydration agents or tracking seal integrity until you have a close call. Once, our lab’s ventilation failed. The next morning, we found more than a dozen bottles, including trifluoromethanesulphonate, with condensation beading up inside. Scrambling to dispose of ruined reagents, all I could think was, a moment of carelessness makes trouble for everyone.
Keep trifluoromethanesulphonate away from water, air, and light. Store it tightly sealed, under an inert gas like argon or nitrogen whenever possible. Label every bottle with open dates and names. Rotate stock, and train every new hand in the lab to recognize the danger. Always store reagents in dry fridges or dedicated desiccators. Relying on “someone else will handle it” invites mistakes.
Better storage protects workers, budgets, and results. Without careful handling, the chemical promises more disaster than discovery. Secure storage isn’t a luxury—mistakes prove it’s a necessity.
Anyone who’s spent time with Tert-Butyldimethylsilyl Trifluoromethanesulphonate—usually shortened to TBDMSOTf—understands the stuff packs a punch. Its main appeal comes from how well it protects alcohols and amines in organic synthesis. That effectiveness comes at a price, because this reagent hits back hard if handled sloppily. Even a moment of negligence leaves you with burns, respiratory trouble, or a ruined experiment. To stay out of trouble, people need to pay attention, not just nod at the safety sheet and move on.
TBDMSOTf doesn’t just irritate. It causes deep chemical burns and chews through organic matter. Splash some on your skin or breathe in its fumes, and you’ll need more than basic first aid. More than once, I’ve seen gloves dissolve from a spill while colleagues rushed to the eyewash after a careless move. Its vapors irritate the nose and throat, and even a little in the eyes leads to serious pain. Don’t let the clear liquid fool you—this isn’t just another bottle in the cabinet.
Goggles don’t just help—they’re crucial. Standard lab glasses leave pathways open for splashes. Face shields offer an extra layer, but at the very least, wrap-around goggles create a real barrier. Ordinary latex gloves break down quickly, so nitrile or something even sturdier works best. Double-gloving helps too, in case the outer glove fails. Long sleeves might sound hot, but exposed arms attract trouble any time, and this chemical doesn’t forgive shorts or T-shirts.
People sometimes underestimate the need for good ventilation. Fume hoods aren’t just for show. Even small-scale reactions can fill the air with harmful vapors. Outside the hood, those fumes linger, sometimes clinging to your clothes. Doors closed, sash low, hands inside: that routine saves a lot of heartache.
No matter how careful you feel, spills still happen. Absorbent pads and proper neutralizing agents should sit within arm’s reach. Using water to clean up this triflate leads to violent reactions and more dangerous fumes. It makes sense to practice neutralizing with sodium bicarbonate or similar milder bases until it’s second nature. Disposal containers marked for strong organosilicon waste help stop accidental mixing, which creates messes no one wants to deal with at the end of a long day.
As someone who’s spent plenty of late nights cleaning glassware and navigating sleeves stuck to bench tops, nothing replaces solid hands-on training. Watching someone else manage their bench, seeing real-time responses to trouble—those lessons stick better than any warning sticker. Rehearsing the emergency shower, eye wash, spill response—every minute spent on those drills lowers the odds of an accident turning into a medical emergency.
Too many assume that hazardous materials only require ticking the right boxes. That attitude leads to accidents. The right gear, regular checks, and respect for the power of TBDMSOTf spell the difference between smooth experiments and visits to the occupational health office.
Every lab worker, from the newest grad student to seasoned tech, deserves an environment where PPE is easy to find, safety equipment actually works, and training keeps up with new processes. Supervisors should feel responsible for setting the tone and stepping in when corners get cut. Sharing stories about close calls isn’t gossip—it’s what keeps hesitation from turning into harm.
Ask anyone in the lab about tert-Butyldimethylsilyl trifluoromethanesulphonate, and you get a spark in their eye or at least a serious nod. Shortened as TBDMSOTf, this mouthful marks its territory in organic synthesis, especially for those safeguarding alcohol or amine groups. Every piece of its name gives away a bit of the structure.
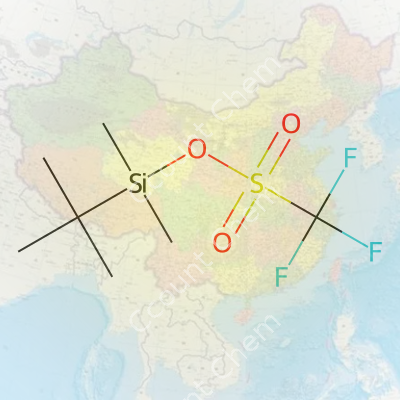
The core pieces are a tert-butyldimethylsilyl group and a trifluoromethanesulfonate, commonly called "triflate." The tert-butyldimethylsilyl (TBDMS) group means a silicon atom sits surrounded by two methyl groups and a branching tert-butyl group—think of it like a silicon wearing armor made of carbon. The trifluoromethanesulfonate part brings a burst of electronegative energy, shaped by a sulfonate group hosting a trio of fluorines.
Shopping for the chemical formula? Here it is, straight and simple: C8H18F3O3SSi.
If you’re hunting for the structure, picture this: The silicon sits in the center, bonded to two methyl groups, one tert-butyl group, and an oxygen which is part of the triflate. The triflate piles on, shaped as CF3SO3, with the sulfur connected to three oxygens (one in a double bond, two single-bonded—the latter extends to the Si center). The silicon, fully surrounded, hands off reactivity and stability across a range of moisture-sensitive reactions.
Chemists draw this with the tert-butyl as a “t-bu” branch, the dimethylsilyl with two methyl “Me” legs, and a chain running to the “OTf” group. Seeing it on paper, you quickly recognize how each group gives the molecule its personality—bulky for shielded reactions, and reactive enough to transfer the silyl unit with ease.
Working in the lab, I lean on TBDMSOTf for its punch in selective protection. Many alcohols or amines hardly flinch in a crowded reaction unless you use something sharp and direct—this reagent delivers that, with its silicon center handling the job where other silyl reagents lag. Moisture is always lurking as an enemy; the triflate anion makes the silyl group transfer much more effective, so reactions finish cleaner, faster, and with far less side product headache.
There’s a real economic and safety angle here. Labs push for higher yields and purity to keep costs and waste low. TBDMSOTf performs under mild conditions, which slashes risk from decomposition or dangerous byproducts that crop up with harsher reagents. It stands as a prime choice when scaling up protection-deprotection sequences in pharmaceuticals and advanced materials.
Despite its power, issues stay on the table. TBDMSOTf’s sensitivity to moisture means careful handling—gloveboxes or dryboxes become standard, even if that slows down workflow. Some chemists keep an eye on cost, too, since triflate reagents don’t come cheap. Solutions keep rolling out: improved packaging, single-use ampules, and greener alternatives all help extend its reach. Sourcing from reputable suppliers with a good track record in purity cuts down on unexpected lab surprises.
TBDMSOTf has shaped a slice of organic chemistry, not because it’s exotic, but because it tackles everyday lab challenges head-on. Recognizing its structure allows chemists to tap into its full value wherever sensitive, selective protection pushes a project forward.
In any organic lab, it’s tough to forget a day wrestling with moisture-sensitive reagents. Tert-Butyldimethylsilyl trifluoromethanesulphonate (TBSOTf) poses one of those real-world challenges. This silylating agent delivers speed and power for protection reactions but meets its nemesis as soon as water lurks nearby. Solvent choice for TBSOTf reactions isn’t about pure convenience—it impacts the final yield, the reaction's cleanliness, and even practical things like work-up.
My hands have seen TBSOTf sizzle on contact with alcohols or sniff out the slightest bit of wetness in poorly dried glass. Accepting that TBSOTf wants nothing to do with water, alcohols, or any traces of acid or nucleophile, most chemists stick with dry, non-nucleophilic, polar aprotic solvents.
A favorite in many labs is dichloromethane (DCM). The reason is clear: dry DCM dissolves the starting materials and keeps the reaction cool. Its moderate polarity helps the silylation run efficiently, and separating the products tends to go smoothly. Tetrahydrofuran (THF) and acetonitrile also rank high. Both lend good solvency for organic substrates, but THF requires extra diligence with drying, since it’s hygroscopic. Acetonitrile gives slightly higher polarity and can handle a broad range of solutes, but it sometimes encourages unwanted side reactions. Keep the molecular sieves handy.
For reactions that run hot or demand stability, toluene often stands in as a reliable substitute. Its non-polar character means it won't assist the electrophilic TBSOTf in the wrong direction, so byproducts don’t creep in easily. Toluene’s higher boiling point also makes it helpful for reactions that need heat.
Ethers like diethyl ether or 1,4-dioxane tempt some chemists. Both look fine on paper, but in practice, they mix with water from the atmosphere and can ruin the whole reaction. Protic solvents—anything with an active hydrogen—just destroy the TBSOTf outright. Alcohols, carboxylic acids, acetone, and DMF spoil the reagent or bring in interference from nucleophiles.
Over time, I learned that small amounts of impurity in the solvent sometimes go unnoticed, but TBSOTf finds them fast. Periodically testing solvents with Karl Fischer titration or by running a trial reaction with a cheap substrate helps catch surprises early.
Routine for me now includes running fresh columns of alumina or molecular sieves on every solvent batch before TBSOTf sits in the flask. If budgets get tight, distilling solvents right before use cuts down on mishaps. Glassware dried at 120°C in the oven makes a huge difference; even a little moisture can tank the whole synthesis.
Sometimes, the reaction's success depends on not just the solvent but the concentration and order of operations. Adding TBSOTf after the substrate dissolves can protect sensitive alcohols from decomposition. Stirring under nitrogen instead of air stops the ambient water from drifting in. For scale-up, working in a glove box isn’t overkill—it’s insurance against those infuriating, low-yield batches.
Choosing the most compatible solvent for TBSOTf has less to do with routine than with discipline—sticking to pure, dry, non-protic environments every time. The detail matters here. TBSOTf keeps its power for those who respect its quirks, and in the right solvent, it turns stubborn alcohols into smooth, protected intermediates in just a few hours.
| Names | |
| Preferred IUPAC name | tert-butyldimethylsilyl trifluoromethanesulfonate |
| Other names |
TBDMS triflate TBDMSOTf tert-Butyldimethylsilyl triflate tert-Butyldimethylsilyl trifluoromethanesulfonate |
| Pronunciation | /ˈtɜːrtˌbjuːtaɪlˌdaɪˌmɛθɪlˈsɪliˌtraɪˌflɔːroʊˌmɛˈθeɪnˌsʌlˈfəʊneɪt/ |
| Identifiers | |
| CAS Number | 69739-34-0 |
| Beilstein Reference | 1915750 |
| ChEBI | CHEBI:87774 |
| ChEMBL | CHEMBL4141313 |
| ChemSpider | 22274844 |
| DrugBank | DB08377 |
| ECHA InfoCard | 44c5b6b3-9b38-44ba-846a-802e96073182 |
| EC Number | 2162-39-4 |
| Gmelin Reference | 721262 |
| KEGG | C19588 |
| MeSH | D017209 |
| PubChem CID | 65602 |
| RTECS number | X78553200 |
| UNII | 5Z86A6SF8B |
| UN number | UN3265 |
| Properties | |
| Chemical formula | C7H15F3O3SSi |
| Molar mass | 290.34 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | Density: 1.214 g/mL at 25 °C |
| Solubility in water | Reacts with water |
| log P | 1.6 |
| Vapor pressure | 4.7 hPa (20 °C) |
| Acidity (pKa) | -2.7 |
| Basicity (pKb) | -4.2 |
| Magnetic susceptibility (χ) | -7.54×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.338 |
| Viscosity | 5 cP (20 °C) |
| Dipole moment | 3.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 410.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS02,GHS05 |
| Signal word | Danger |
| Hazard statements | Hazard statements: H314, H302 |
| Precautionary statements | Precautionary statements: P210, P261, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-3-1-W |
| Flash point | 40 °C (104 °F; 313 K) |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 2000 mg/kg |
| NIOSH | NA9100000 |
| PEL (Permissible) | 400 mg/m³ |
| REL (Recommended) | 0.02 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Trimethylsilyl trifluoromethanesulfonate Triethylsilyl trifluoromethanesulfonate Tert-Butyldiphenylsilyl trifluoromethanesulfonate |