A few decades back, people in chemical labs went after new materials that could withstand both time and pressure. They needed polymers that wouldn’t buckle under the stress of modern industry. Sulphoethyl methacrylate cropped up alongside the rush for specialty acrylics. Back then, researchers mixed up its first batch hoping to strike a balance between toughness and flexibility. As folks tinkered with acrylates, adding different functional groups, the sulphonate group stood out. It let chemists dial in better water compatibility compared to plain methacrylates. The old journals read like a real diary of trial and error, with every formula scribbled in the margin. Over the years, the compound joined a family of additives shaping everything from resins to hydrogels, earning itself a spot in textbooks and patents. No one stumbled on it by accident; it came from the real persistence that defines how applied science moves forward.
You open a container of sulphoethyl methacrylate and find a pale, viscous liquid or sometimes a waxy crystalline mass, depending on the room’s temperature. The odor sits sharp and faintly chemical, a telltale sign of methacrylates in general. In labs and factories, it serves as a reactive monomer, a building block for all sorts of performance materials. Chemists value it for the sulphoethyl group, which brings a remarkable level of ionic charge to backbone polymers. As a result, it lands in formulation sheets for everything from contact lenses aiming for more comfort to membranes needing more selective permeability. Companies label it under different names or codes, but they all chase the usability tied back to its methacrylic skeleton combined with the sulphonate side chain.
In the real world, numbers matter. Sulphoethyl methacrylate comes with a molecular weight around 210 g/mol, a density flirting with 1.22 g/cm³, and a moderate boiling point that usually stays out of reach unless you push hard. The material dissolves well in water, thanks to the sulphonate group, but doesn’t resist aggressive acids or bases forever. Because of the double bond in the methacrylate part, it polymerizes quickly with the right initiator. This tendency can cause real headaches if you forget a stabilizer in storage—chemists have lost entire batches to runaway reactions. Its refractive index sits close to 1.48, which ends up helping when precise light transmission matters, like in optics or filtration devices. Among acrylic monomers, this one stands out not because it’s flashy, but because it covers a useful range of behavior—right between the stubbornly hydrophobic and the super-soluble.
Manufacturers stamp a typical assay of over 97%, with color measured on the APHA scale, aiming below 50 for premium grades. Chloride residues hold tight below 0.1%, and polymerization inhibitors keep moisture low for longer shelf life. Data sheets read C6H10O5S as the chemical formula. Labels include safety codes about irritation, and shipping stays regulated by standard hazmat protocols—UN numbers, hazard classes, and all. The risk grows real when bottles sit unprotected, especially in heat. Good suppliers add both batch numbers for traceability and clear expiry dates. Real-world quality checks involve running NMR or IR, making sure there’s no off-profile impurity lurking from production. Folks in the field look for clear, transparent product—yellowish tint or particulates give reliable warning that something’s gone off-spec or started to polymerize too soon.
Producing sulphoethyl methacrylate takes more than pouring two liquids together. It usually starts with methacrylic acid or its methyl ester. These react with 2-chloroethanesulfonic acid sodium salt under alkaline conditions. The substitution carves out the sulphoethyl group, working best in solvent systems like acetonitrile or DMF. The post-reaction mix has to be purified, not only by extraction but by distillation under high vacuum to dodge thermal decomposition. Anyone in the lab knows the smell of acrylics and the sting in your nose when it goes wrong. Large-scale plants run continuous flow processes, but even there, folks watch temperature and pH like hawks. Sloppy filtration or incomplete separation, and you end up with a mess of byproducts. Crystallization and washing bring the product up to accepted purity, though every chemist can tell you some days the yield just doesn’t line up with the textbook promises.
This compound doesn’t just sit on a shelf. Lab teams take the double bond and react it with free radical initiators, chaining the sulphoethyl group into bigger polymers. The sulphonate can also undergo counterion exchange, swapping sodium for potassium or even organic cations to tweak solubility. Post-polymerization, craftsmen graft the monomer onto polymer backbones or insert it into copolymers with styrene, acrylamide, or other methacrylates. The most interesting outcomes turn up in hydrogels, where the sulphoethyl adds ionic strength and channel-like porosity. Some use it as a crosslinking co-monomer, adjusting physical properties without losing the hydrophilicity that makes sulphoethyl methacrylate so handy. Modifications depend on the final target: medical devices, water filters, smart coatings.
Search literature or catalogs and you might find the same stuff under names like SEM, 2-sulfoethyl methacrylate, or sodium 2-methacryloyloxyethylsulfonate. Some suppliers push specific trademarks, but the scientific literature uses those synonyms liberally. This doesn’t confuse seasoned buyers, but newcomers often end up cross-checking CAS numbers to stay on track. In English, Russian, Japanese, the core identifier doesn’t change much—it’s the sulphoethyl group that’s the star. For regulatory filings, the full IUPAC name gets its run, mostly for import/export paperwork and patent claims.
Folk handling sulphoethyl methacrylate know enough to keep gloves handy and bring out the goggles. The liquid stings if it hits bare skin, and inhaling vapors is not on anyone’s list of smart moves. I’ve seen new lab workers get careless and pay for it with an itchy rash—nothing life-threatening, just plain unpleasant. The monomer isn’t flammable at room temperature, but its vapors can irritate. Teams store it under nitrogen or argon, cool and in the dark, dodging both runaway polymerization and light degradation. Spills call for quick action with absorbent pads and thorough ventilation. Waste disposal heads straight for the hazardous waste bins, no shortcuts. Factories working large-scale batches layer on extra containment, scrubbers, and real-time monitoring for leaks, especially in closed system operations.
One day, sulphoethyl methacrylate lands in a water desalination membrane; the next, it shows up in a soft hydrogel for drug delivery. Its strong suit is blending water compatibility with structural stability, letting engineers tune both flow and strength. In the world of contact lenses, manufacturers rely on its ability to hydrate without swelling out of control. Water purification systems pick it for building membranes that screen out ions without getting fouled by biofilms. In coatings, it creates anti-static finishes. Some specialty adhesives look to it for flexibility in wet environments. I’ve chatted with engineers who rely on these materials to meet demanding specs—few other monomers offer this blend of ionic character and processing flexibility.
Academic labs and R&D teams keep probing ways to spin new value from sulphoethyl methacrylate’s structure. There’s talk of smart hydrogels that change in response to pH and salt, aimed at targeted delivery in medicine. Others mix it into block copolymers chasing better proton conductivity for fuel cells. I once attended a conference where teams explored nanoscale self-assembly using this monomer, pushing boundaries in both electronics and biomedicine. The push comes from customers demanding membranes that filter more efficiently or gels that mimic human tissue more closely. Ongoing grants fund toxicity screening, especially with a view to scaling up medical uses, since new regulations make no room for shortcuts. Chemists tinker with surface modifications, thinking about fouling resistance and compatibility with cells, all aiming for the next patent or commercial breakthrough.
No chemical should enter mass markets without a hard look at long-term exposure. Toxicity studies show that sulphoethyl methacrylate behaves much like other low-molecular-weight acrylates—some risk of skin and eye irritation, but not a notorious mutagen or carcinogen. Rats exposed to high doses pull up mild inflammation, but there’s no strong evidence for chronic toxicity at occupational levels. The Environmental Protection Agency keeps tabs on it precisely because of its water solubility and use in consumables. Medical device makers run extra cytotoxicity panels, making sure polymers made from it shed no dangerous monomers over years of use. For all its growing adoption, the industry keeps pointing to regular health checks, improved ventilation, and protective training as front-line defenses.
The market for functional monomers only grows as new industries crave smart materials. Sulphoethyl methacrylate stands a good chance of powering the next wave of biocompatible hydrogels or tough-but-soft coatings. If manufacturers solve scale-up purity and cost issues, this compound could show up in everything from medical wearables to next-generation desalination plants. Regulatory changes could nudge further research into non-toxic alternatives, but so far, the field treats it as a key workhorse. Industry insiders watch researchers experiment with digital manufacturing and greener synthesis routes. The coming years will see more public-private partnerships, especially as the health sector hunts for safer, more flexible polymers.
No one asks about chemicals like sulphoethyl methacrylate at a party, yet it pops up in more places than you might guess. Chemists see it as a building block in designing advanced materials. Behind the curtain, sulphoethyl methacrylate creates real change in industries shaping the gear and tools used every day.
Having struggled with dry contact lenses during allergy season, I've learned the hard way that hydrogels make a difference. Sulphoethyl methacrylate gets used to create hydrogels in medical devices and wound dressings. The molecular structure draws in water, so hydrogels based on this chemical stay softer and hold more moisture on the eye. That means fewer headaches for people seeking long-lasting contact lens comfort and fewer dried-out bandages for wounds. In the lab, these materials help mimic natural tissue, offering tools for research and treatment at the same time.
Anyone who’s painted a deck knows the pain of chips and peeled surfaces. Adding sulphoethyl methacrylate to acrylic polymers doesn’t just sound fancy — it delivers coatings that cling better, shrug off water, and last longer on tough surfaces. It adds robustness and makes coatings less likely to break down under UV light and wear. These characteristics translate to lower repair costs and less waste from constant reapplication, things that matter to both manufacturers and homeowners.
Try spending time in a region with hard water, and you’ll see why water softening technology matters. Sulphoethyl methacrylate’s side groups make it good for producing resins used in ion exchange. These resins help strip out calcium and magnesium ions, improving not just the taste but also the lifespan of appliances and pipes. Stronger, more efficient ion exchange resins mean cleaner water with less downtime. Reliable water treatment connects straight to public health — fewer minerals, fewer clogged pipes, fewer headaches for everyone.
Builders in the world of fuel cells and batteries also count on specialized polymers. Sulphoethyl methacrylate helps form membranes with strong ionic conductivity, which means batteries and fuel cells last longer and perform better. I’ve read how researchers push for smaller, cheaper, and greener power sources, and these advances only matter if materials like these deliver real-world performance.
Sulphoethyl methacrylate needs respectful handling. People exposed to it over time can face skin and eye irritation. Chemists and factory workers benefit from clear labeling, protective gear, and ventilation. Knowing the risks makes innovation safer. Moving forward, finding safer alternatives or improving protective standards will keep both workplaces and end users safer, especially as use grows.
From hydrogels to protective coatings, sulphoethyl methacrylate shows up where toughness, flexibility, and water-handling set the bar higher. Recognizing where these chemicals fit in the larger puzzle helps everyone from consumers to industry leaders make smarter choices. Better material science makes life easier, whether that’s reaching for a contact lens, filtering water, or powering clean devices. For anyone building the next generation of useful products, understanding chemicals like this unlocks new, workable solutions.
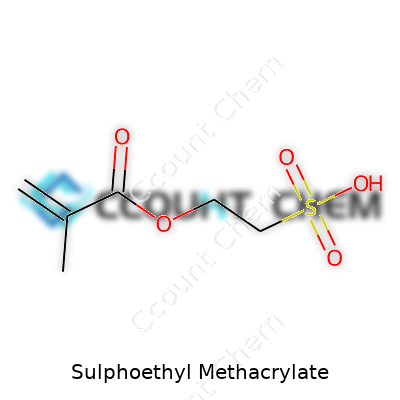
Sulphoethyl methacrylate keeps showing up in specialty polymer spaces for a reason. Its molecular design brings together two main features: a methacrylate group, famous for its ability to form strong, durable plastics, and a sulfonic acid group, which sets it apart through its water-loving nature. Anyone who has worked with acrylics notices the difference once charged groups enter the scene. The result: Sulphoethyl methacrylate can introduce ionic functionality into polymers in a way that regular methacrylates can’t touch.
My experience with water treatment membranes and specialty coatings has shown that materials with sulfonic acid groups bring one huge advantage to the table: water compatibility. Sulphoethyl methacrylate dissolves well in water, giving it flexibility as a building block. Once incorporated in a polymer, it contributes negative charges on the surface or backbone. These charges attract positive ions from water—an effect that forms the basis for ion-exchange resins and certain superabsorbent applications.
Backed by published research, polymers containing sulphoethyl methacrylate improve performance in electroconductive coatings and controlled drug release. That’s all thanks to the ability of the sulfonic group to host and exchange ions, which also helps in fuel cell membranes and anti-static fabric finishing.
Plastics or resins made from simple methacrylates can fall short in high-durability coatings or adhesives. Adding sulphoethyl methacrylate into a formulation often leads to better adhesion to glass, metal, or mineral fillers. The reason: The charged sulfonic groups interact with surface ions or hydroxyls on those substrates. In my work with composite materials, a little bit of this monomer can make a vast difference in sticking power and resistance to wear.
Anyone working with paints or pigment dispersions pays attention to how additives affect particle stability. Sulphoethyl methacrylate shows up here too, helping disperse pigments in waterborne systems. Its ionic character prevents pigments from clumping, which saves a lot of headaches in ink and paint formulation.
Adding a sulfonic acid group sounds like it might weaken a polymer, but sulphoethyl methacrylate shows it’s not that simple. When used in the right proportion, it can actually boost flexibility and control swelling in water, as well as offer moderate resistance to heat. You don’t want to overdo it, since too much can lead to water uptake and softening. The right balance gives membranes that are tough yet responsive, a feature that gets used in filtration and battery separators.
Every chemical used in manufacturing deserves a discussion on safety and environmental impact. Sulphoethyl methacrylate carries a low-to-moderate toxicity profile. Working with it in the lab, I’ve learned good ventilation and gloves are non-negotiable. On the plus side, its solubility and degradability can support more environmentally sensitive design in water treatment or medical disposables, offering a potential edge for sustainable chemistry.
Innovation in polymers points toward functionality rather than brute strength alone. Sulphoethyl methacrylate proves that a well-placed charged group changes everything—from ion-exchange and conductivity to better adhesion and pigment stability. The challenge falls on chemists and manufacturers to keep improving formulations and handling, to unlock the full benefits of this useful monomer.
Most people see chemical names like sulphoethyl methacrylate and feel a wave of uncertainty. The name alone sounds intimidating. It shows up in the building blocks of polymers you’d find in paints, adhesives, and plastics. On paper, it’s a material chemists like for its toughness and flexibility. For those of us outside the lab, questions about its safety pop up fast, especially if it ends up in everyday goods.
A direct splash of sulphoethyl methacrylate in the eyes or on the skin can sting or burn. Fact is, this chemical belongs to a family known for causing irritation. Redness, itchiness, and even blistering can follow uncontrolled contact. If the liquid hits bare skin for long, discomfort grows. Many painters and factory workers wear gloves and goggles, not just for show, but because they’ve learned the hard way.
The Material Safety Data Sheet warns about possible allergic reactions after repeated or prolonged use. Once in a while, someone builds up sensitivity, which means patchy rashes or swelling start to show up even when the dose is small. My own hands have reacted to acrylic monomers before, so I can vouch for just how aggravating that feeling is.
Inhalation creates a different set of risks. Freshly made sulphoethyl methacrylate can release a strong odor. Breathing too much of the vapor often leads to headaches, dizziness, and a raw throat. Working in a cramped or poorly-ventilated area makes things worse. I once helped install industrial flooring with a sister compound; we skipped masks, paid for it with scratchy throats all week.
Short bursts of exposure usually resolve once someone steps outside. There’s less evidence of lung damage over the long term, but that doesn’t excuse carelessness. Chemical exposure doesn’t need to reach crisis levels before it disrupts health and focus at work.
Sulphoethyl methacrylate stays low on the acute toxicity scale. Accidental swallowing causes more immediate irritation than systemic organ damage. It’s not as hazardous as strong acids or heavy metals, but it doesn’t get a free pass. Birds and fish don’t do well when traces collect in waterways, which worries anyone who’s seen the aftermath of a chemical spill. Good chemical protocols are more than legal paperwork—they protect local water and keep communities safe.
Most responsible manufacturers work with closed systems and trained staff. From a health and safety perspective, it’s not about hiding ingredients—it's about controlling them. Community safety groups push for stricter rules for storage and disposal, for good reason. There’s nothing minor about finding chemical traces down river from an industrial site.
The smartest action involves clear labeling and personal protective equipment. It helps lab workers, janitors, even artists avoid the kind of exposures that spark long-term resentment—or lawsuits. In my own projects, switching to safer, water-based polymers proved possible. Sometimes the performance isn’t identical, but health trumps smooth finishes.
With some chemicals, there’s a clear line between risk and recklessness. Sulphoethyl methacrylate sits on that line for many industrial users. An ounce of prevention keeps employees healthy, floors cleaner, and riverbanks chemical-free. In the end, informed choices and sound habits steer us away from trouble, toxic or not.
Anyone using sulphoethyl methacrylate in a lab or plant has seen warning labels and safety data sheets listing out lots of technical advice. At the core, what really matters is that this chemical goes south pretty quickly if someone gets casual with it. Leaving it on the wrong shelf or exposing it to sunlight could spark more trouble than most folks expect. Overheating or moisture creeping in can alter its chemistry, and the wrong container sparks an expensive lesson. Having worked with similar monomers in industrial polymerization, I’ve seen too many costly spills caused by plain old carelessness.
Most folks aim for “cool and dry” like it’s just a checklist item. For sulphoethyl methacrylate, that’s not just bureaucracy talking. Warm storage spaces accelerate unwanted reactions, creating dangerous byproducts or ruining expensive supplies. Any hint of water entering the drum or bottle can start a reaction. Just a damp storeroom gets risky because humidity, even in small bursts, can open the door for polymerization inside the container. Sunlight does its own mischief by breaking down the material over time. It isn’t just about wasted money—a runaway reaction in a warehouse can put people at real risk. Facts from chemical hazard reports back this up: improper storage has been documented as leading to fires, pressure buildups, and inhalation injuries.
Plastic and glass both seem fine at first glance—until you remember that sulphoethyl methacrylate reacts with incompatible metals and will eat through cheaper containers over time. Sealed glass bottles with tight-fitting lids keep the chemical in check. Containers should be left in a stable spot, nowhere near acids, oxidizers, or open flames. My former lab made a basic but critical habit out of storing all reactive chemicals on a dedicated shelf, away from regular traffic. The people who skip that step often end up regretting it.
No label, no excuse. Sulphoethyl methacrylate, stored improperly and unlabeled, turns into a safety lottery. Examples from the American Chemical Society show many accidents come down to someone grabbing the wrong bottle because someone else didn’t bother writing things down. It doesn’t take special training to understand plain words taped to a jar—just respect for the next shift coming in.
It’s easy to toss another chemical up onto a shelf and call it a day. The difference between an incident and a safe lab environment comes down to habits and clear standards. Regular checks for leaks, reviewing expiration dates, and keeping an eye out for changes in color or consistency make a world of difference. In my own work, the worst days came from skipped checks or hurrying past safety routines.
Real progress comes from a few practical steps: use sealed, labeled glass containers, keep things dry, never ever store next to heat, acid, or flame; teach every new staff member the story behind every rule. Encouraging questions and running regular storage audits removes the “I thought it was fine” excuse. That’s how risks shrink and expensive lessons stop repeating themselves.
Factories and labs working with advanced plastics and hydrogels often keep Sulphoethyl Methacrylate on hand. This monomer doesn’t turn heads, yet its involvement can make a big difference in the feel and performance of everyday products. I’ve seen it pop up during visits to manufacturing floors and polymer research centers. People use it because it brings a balance of water-friendliness and strong bond formation that’s tough to match.
Companies treating water have a soft spot for this stuff. It helps shape resins for ion exchange, making water purification more reliable. The material gets used to pull out minerals and even some nasty metal traces that sometimes sneak into municipal and industrial systems. Everyone depends on clean water. If you sit in on a planning meeting at a water works, you’ll likely find Sulphoethyl Methacrylate in the notes. The technology allows for tighter control over which unwanted bits get yanked out of the stream.
Hospitals and clinics rely on diagnostic devices, contact lenses, and controlled-release drug systems made with precision. Bioengineers look for monomers that play well with the human body—no one likes terrible side-effects from their eye drops or medical tests. Sulphoethyl Methacrylate makes polymers that hold water, stay clear, and resist breaking down too soon. You’ll run into it in the guts of blood separation columns and filters designed to trap only what truly matters. From conversations with chemists and technicians, the focus always comes down to reliability and making things that patients never have to think twice about using.
Paper mills don’t get much attention, even though they’re constantly searching for ways to keep paper crisp and strong from the press to your desk. A key ingredient for specialty coatings, Sulphoethyl Methacrylate helps papers grab onto inks better and resist smudges. Fabric technologists borrow similar tricks, blending the monomer into coatings for moisture management and anti-static properties in athletic apparel. These improvements might sound minor, but on the production line, even tiny boosts in print quality or textile comfort can mean fewer returns and more loyal buyers.
Factories molding circuit boards or laying out sensors for cars and phones can’t tolerate much impurity or sloppiness. Sulphoethyl Methacrylate enables chemists to dial in surfaces just right, so circuits don’t short and sensors stay sensitive. Research labs keep exploring what happens if you swap this monomer into display panels, membranes, or adhesives—there’s steady demand for tougher, lighter, and smarter components. The hunger for smaller, more reliable gadgets pushes these materials to their limits, and monomers like Sulphoethyl Methacrylate support those goals.
Not every industry moves at the same speed. While some openly tinker with new uses for this monomer, others stick to old recipes and patterns. That opens space for startups and research teams to look at greener ways of producing and recycling these materials. Proper handling matters, as any lab manger will tell you. Focusing on safe practices and smarter waste management makes a big difference down the road, both for workers and the environment. Better training, investment in cleaner reactors, and support for circular supply chains lay a stronger foundation. Every step toward smarter chemistry gives industries a way forward, balancing innovation with real-world needs and risks.
| Names | |
| Preferred IUPAC name | 2-methylprop-2-enoic acid 2-sulfoethyl ester |
| Other names |
2-Methyl-2-propenoic acid, 2-sulfoethyl ester Sulfoethyl methacrylate 2-Sulfoethyl methacrylate 2-Sulfoethoxy methacrylate Methacrylic acid 2-sulfoethyl ester |
| Pronunciation | /ˌsʌl.foʊˈiː.θəl mɛˈθæk.rɪ.leɪt/ |
| Identifiers | |
| CAS Number | 10595-80-9 |
| Beilstein Reference | 1718730 |
| ChEBI | CHEBI:52819 |
| ChEMBL | CHEMBL169786 |
| ChemSpider | 72814 |
| DrugBank | DB13972 |
| ECHA InfoCard | ECHA InfoCard: 03c0f6e4-8b24-4021-a6b9-1ba48a887ffe |
| EC Number | 212-426-2 |
| Gmelin Reference | 85939 |
| KEGG | C18851 |
| MeSH | D017350 |
| PubChem CID | 17817 |
| RTECS number | GV7870000 |
| UNII | I6P7H7G7B2 |
| UN number | 《UN3082》 |
| CompTox Dashboard (EPA) | DTXSID5020436 |
| Properties | |
| Chemical formula | C7H12O5S |
| Molar mass | 210.22 g/mol |
| Appearance | White crystalline powder |
| Odor | Characteristic |
| Density | 1.282 g/cm³ |
| Solubility in water | soluble |
| log P | -2.0 |
| Vapor pressure | 0.01 mmHg (20°C) |
| Acidity (pKa) | pKa = 1.5 |
| Basicity (pKb) | 6.05 |
| Magnetic susceptibility (χ) | -7.77e-6 cm³/mol |
| Refractive index (nD) | 1.464 |
| Viscosity | 10-20 mPa.s |
| Dipole moment | 4.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 323.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P333+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-2-2 |
| Flash point | > 113°C (235°F) |
| Autoignition temperature | 430°C |
| Lethal dose or concentration | LD50 Oral Rat 9770 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 8700 mg/kg |
| NIOSH | NIOSH: RV1150000 |
| PEL (Permissible) | Not established. |
| Related compounds | |
| Related compounds |
Methacrylic acid Ethyl methacrylate 2-Hydroxyethyl methacrylate Sulphopropyl methacrylate Methacryloyl chloride |