Chemistry classes used to buzz about acids like sulfuric and hydrochloric long before anyone at my local lab even mentioned sulphamidic acid. Yet, by the late 19th century, scientists began tinkering with compounds where a sulfur atom hooks up with both an amide and a hydroxyl group. This curiosity led to the birth of sulphamidic acid, also written as sulfamic acid. As time rolled forward, industrial specialists discovered its knack for cleaning scale and acting as a middleman in producing dyes and sweeteners. It didn’t find fame overnight—researchers took decades to unlock its uses, one experiment at a time, always with the goal to shape something safer or more resourceful than the acid tools of the previous century.
The world often recognizes sulphamidic acid as a white, non-volatile, crystalline solid. No strong scent wafts up from an open package, and powders or granules tend to stay dry unless left uncovered in damp air. Factories ship it in sacks or drums labeled with hazard symbols, underscoring its potential to harm when mixed with water or bases, and for good reason—mistakes around storage or usage have burned more than one set of engineering hands. Large-scale production makes this compound widely available in technical and food grades, and suppliers stamp each batch with precise assay percentages, reflecting growing regulatory scrutiny. For most buyers, knowing whether the acid contains traces of heavy metals or other contaminants ranks as a dealbreaker, especially for pharmaceutical or food applications.
Folks expecting drama from this acid often walk away surprised. Sulphamidic acid, or H3NSO3, delivers a melting point near 205°C, not that you’d ever want to heat it that high unless you’re running a reaction vessel with good ventilation. The compound dissolves well in water, giving a slightly acidic solution—useful for precise pH adjustments or cleaning circuits. It skips the showy fizz with bases, working more like a slow-release cleaning agent, steady as it neutralizes lime deposits. It stays relatively stable if left dry and cool, but add moisture or strong bases and it speeds into breakdown territory, generating ammonia gas and sulfate salts. In my experience, too much heat or sunlight can set off slow decomposition, which means you never want a stockroom reaching tropical temperatures.
Manufacturers set their sights on purity when defining sulphamidic acid specs. Labels spell out assay percentages—95% and above for technical grade top the list—followed by allowable levels of moisture and insoluble matter. Regulatory agencies want explicit warnings about eye and skin irritation, flammability, and emergency measures. I’ve handled containers stamped with GHS hazard pictograms, which demand gloves and goggles before you even open the bag. Product names on documentation include both “sulphamidic acid” and “sulfamic acid” due to regional spelling quirks, but both point to the same molecular formula. Barcodes, batch numbers, and manufacturing details help trace problems right back to the source, adding a sense of accountability missing in chemical supply chains just a generation ago.
Mixing urea with fuming sulfuric acid catapulted this chemical into mainstream production. In the lab, I’ve watched tannish clouds rise as reactants meet, cooling the mixture to bind the heat. Industrial setups flow the gases through water, condensing the vapors into solid sulphamidic acid. Process tweaks, like changing the acidity or temperature, influence both the speed and quality of the product. Controlling byproducts, such as ammonium sulfate or excess water, remains a game of balance—and clean workstations stave off dangerous reactions that leave toxic residues. The ACHEMA trade fairs I attended showcased new, greener synthesis approaches where less energy gets wasted, proving that the industry never stops refining age-old recipes.
Sulphamidic acid shows a versatile profile on the lab bench. Combine it with oxidizing agents and it can morph into nitrogen gas, making it handy for removing nitrites or other reducing agents from chemical streams. In organic synthesis, I’ve seen the compound shift into new forms through N-alkylation or esterification, opening pathways to specialty dyes and sweeteners. This flexibility means that reaction conditions—whether a scientist adds heat, switches solvents, or increases acidity—change both the products and risks. Handling it safely always means treating it as more than a cleaning powder; with the wrong ingredients, it can unleash violent fumes or destroy reaction vessels.
No matter which catalog I flip through, the aliases keep stacking up. Sulfamic acid, amidosulfonic acid, and amidosulfuric acid show up the most. Imported shipments from Europe sometimes call it sulphamidic acid, while North American contracts usually default to the spelling without the “ph.” Cleaners and descaling agents often relabel it as “descaler powder” for mass-market consumers, yet chemical buyers never stray beyond the standardized nomenclature. This tangle of product names often causes confusion at customs if documentation slips, so robust digital tracking matters just as much as the actual purity.
Too many stories circulate about workers skipping gloves or face shields around sulphamidic acid. Once it touches skin or eyes, irritation sets in fast, and inhaling the dust can cause serious respiratory problems. I've seen folks scramble to an eyewash after a careless scoop sent powder swirling. Whether tackling scale buildup in boilers or making dyes, I stick to labs fitted with proper airflow controls and enforce emergency drills if a spill occurs. OSHA and EU-safety rules treat this acid seriously, requiring up-to-date Safety Data Sheets and routine training. Insurers keep a close eye on storage conditions: sealed drums, away from anything alkaline, dry, and shielded from direct light. Packaging gets color-coded to warn of risks, and I push for regular inspections—complacency has a price in chemical warehouses.
The acid found its place early on in cleaning, stripping away hard-water scale from boilers and pipes. Next stop: the pulp and paper sector, where it zapped out stubborn mineral stains. Later, I worked with textile mills that adopted sulphamidic acid for preparing fibers before dyeing, chasing brighter and longer-lasting colors. These days, food engineers sometimes use it in sweetener synthesis, although purity rules get even stricter. Swimming pool technicians rely on it to control pH and clean filters. Dentists test it for gentle enamel cleaning, and electronics factories count on it to wash metal surfaces without roughening them. Right across these industries, efficient performance always bumps against strict safety controls—a dance that demands alert staff and sharp procurement practices.
Academic journals keep turning out studies exploring sulphamidic acid as a reagent for new molecular frameworks or catalysts. Teams experiment with changing substituent groups, aiming for products that work faster or handle higher temperatures. In water treatment, scientists are probing ways to recycle sulphamidic acid by-products, lowering plant emissions. Collaborations between universities and chemical giants spark patents pitched at greener manufacturing, shifting from high-heat synthesis to low-energy routes. Digital monitoring platforms track product quality, saving trial-and-error time, and open-source chemistry databases reveal how tweaks at the molecular level shape downstream performance. After years in labs and at conferences, it’s clear that R&D shapes more than commercial margins; it sets new safety standards and guides regulatory agencies.
Experiments with rats and rabbits taught early toxicologists that ingestion causes kidney and bladder irritation, but low doses get flushed out by healthy renal systems. Skin contact tests report irritation, never systemic harm unless exposure goes unchecked for days. Some agricultural scientists studied environmental effects, finding that aquatic life suffers if run-off collects too heavily—enough reason for wastewater treatment plants to implement tight discharge controls. No one uncovered evidence linking sulphamidic acid to cancer, and workers with years of exposure under monitored conditions rarely suffered major incidents. Still, every time someone developed a lingering cough or rash, company doctors traced it to improper gear or inadequate ventilation. Toxicity studies helped the broader industry push lawmakers to raise documentation and labeling standards, a win for workers and downstream users.
Sustainability trends shape the roadmap for sulphamidic acid. Demand for less energy-intensive manufacturing pressures companies to cut emissions and boost recycling of process waters. Biotech startups are investigating enzyme-driven synthesis to sidestep harsh conditions. In my field, talk often drifts toward “greener” blends or developing better neutralizer agents for safer lab cleanups. Analytical chemists build faster, more selective detectors to spot residual acids in food and water—public health agencies keep an eye on this, knowing mistakes can ripple from the factory floor to family kitchens. The regulatory environment grows tighter, tracking both workplace exposure and downstream risks. Industrial training shifts from rote learning toward hands-on simulation, coaching workers how to spot hidden dangers. Keeping sulphamidic acid both accessible and safe stands as a shared commitment, linking inventors, supply managers, and safety engineers around the world.
Sulphamidic acid, sometimes called sulfamic acid, pops up in places you may not expect. This compound gets tossed into everyday cleaning products for a good reason. Picture the stubborn limescale clinging to your coffee machine or hiding inside industrial boilers. Using tap water over and over leaves hard deposits behind, clogging pipes and slowing down equipment. Sulphamidic acid breaks down that crusty buildup fast, clearing it out safely without clouding up surfaces or leaving behind sticky chemicals. Supplies that tackle deep cleaning—from descalers for kettles and dishwashers to bathroom tile sprays—often owe their strength to this acid’s punch.
Paper mills tell a quieter story about sulphamidic acid. The pulp they use to make notebook pages or cardboard needs to be just right, or the whole process bogs down. This is where this acid steps in, adjusting pH and helping remove leftover wood resins. Without it, paper might come out blotchy or even fall apart when wet. Staff in mills see how a steady hand with sulphamidic acid makes the difference between stacks of unusable sheets and crisp white paper ready for books or printing press runs. Reports from production lines show that even small changes in acid concentration affect fiber bonding, making this compound an everyday necessity for achieving quality at scale.
Anyone who relies on municipal water owes a quiet thanks to compounds like sulphamidic acid. Water treatment plants use it to control scale-forming minerals and keep equipment running more efficiently. The gear and pipelines in these systems stay cleaner and last longer, which staves off costly replacements and helps prevent unexpected cancellations in service. What’s more, researchers have noted that sulphamidic acid can break up the chemical bonds in traces of heavy metals, reducing some of the risks lurking in older water systems. Any improvements here represent more than just infrastructure upgrades—they help neighborhoods count on safe water from their taps.
Of course, handling chemicals calls for clear guidelines, and sulphamidic acid is no exception. Splashing this acid around without gloves or a mask burns skin or irritates eyes fast. Factories using large amounts set out hazard training for their employees, along with emergency rinse stations and warnings about fumes. Government agencies like the EPA and OSHA dig into industry usage every year, tracking health and waste disposal to make sure practices protect both people and waterways.
Looking at the bigger picture, researchers are working on ways to recycle spent sulphamidic acid from industry—closing the loop now means less runoff into rivers and less energy spent on manufacturing fresh batches. Some labs have reported promising results using natural minerals as catalysts, which cuts down the need for harsh chemicals while keeping the cleaning power that manufacturers need. These small, careful changes add up.
Few substances pack as much practical punch as sulphamidic acid. Our coffee tastes fresher because kitchen machines stay unclogged, paper on store shelves holds up better because mills can fine-tune their processes, and water authorities stretch budgets further thanks to reliable pipes free from corrosion. As more companies and researchers look at safety and ways to shrink the environmental footprint, sulphamidic acid keeps finding a place, both on shop floors and behind the scenes in daily life.
Sulphamidic acid, known in many labs simply as sulfamic acid, pops up in schools, workshops, and factories. Its popularity comes from its strength as a cleaning agent and descaler. Plenty of us grab it for clearing out tough limescale or prepping metal surfaces. Still, before anyone tears open a bag or dumps a scoop in a tank, it’s worth taking stock of its safety profile.
Despite its humble appearance—a white, grainy powder—sulphamidic acid can stir up trouble fast. Get some on your hands, and things start to itch or burn. Breathe in the dust or catch a cloud of powder, and your throat will feel it. Eye splashes make for a miserable afternoon. According to the National Institute for Occupational Safety and Health (NIOSH), direct contact can trigger both skin and respiratory irritation. Anyone who’s knocked over a jar knows the sting: the initial wash in the eyes, then red, watery irritation for hours.
Mixing it with other cleaners, especially those containing bleach or strong bases, causes even more problems. Sulphamidic acid releases toxic fumes if it meets chlorinated products. Too many DIY attempts at “super cleaners” end up with folks hacking and gasping in poorly ventilated rooms.
Workshops and high school chemistry teachers often show how quickly it dissolves mineral buildup. I remember the first demonstration in a school lab. A crusty kettle returns to a shiny state in minutes. Yet, scattered powder on a desk or a lingering puff in the air proves why proper habits matter. Eye protection gets ignored; gloves disappear after a hasty hand wash. Rapid jobs and shortcuts invite accidents.
At home, it’s tempting to scoop and scrub without a second thought. Few folks read the label. The Consumer Product Safety Commission clocked up several reports of minor chemical burns and trouble breathing each year from household use. The stuff is powerful, and a splash won’t just clean a tile; it can pit metal or dull surfaces where it sits.
Respect starts with the basics. Keep gloves nearby, slap on goggles, and work in a spot with real airflow—not just a cracked window. Labeled containers deserve their reputation: stray powder in an unmarked jar could end up in the wrong hands or cart, inviting an accident. A dust mask or respirator doesn’t make anyone look cool, but anyone with a cough after breathing the dust knows it saves lungs.
Storage matters, too. Keep the powder dry, sealed tight, and clearly marked. Standing water, old bottles, and leaky bags turn a benign powder into a serious problem. Training and reminders never go out of style: a quick safety sheet printed near a workbench saves headaches. In any shared space, making safety a habit cuts down on those “I thought it was sugar” moments.
Sulphamidic acid’s power carries clear responsibility. Workers and hobbyists need solid habits and good information to stay safe. Clear labeling, basic training, and simple precautions don’t just keep people safe; these steps keep the focus on getting work done, not cleaning up after accidents. Learning from past close calls in schools and homes, a little respect for this powder goes a long way.
Sulphamidic acid tends to show up in industrial cleaning, metal picking, and sometimes even the paper industry. Folks working in those environments need to stay alert—the stuff can cause real trouble if handled the wrong way. My own time in a chemical plant taught me that ignoring the fine print on storage guidance creates more headaches and safety issues than anyone wants to deal with. One misstep leads to damaged containers, burned skin, or toxic air.
Sulphamidic acid pulls in water from the air like a magnet, which changes its chemistry and eats away at packaging. I remember a crew left a barrel loosely capped in a humid loading dock for just a day. The resulting sticky mess clumped up inside, making it unmeasurable and unsafe for use. So tight sealing is more than a suggestion, it’s the reality in any warehouse or storage room. Metal drums or heavy-duty polyethylene containers with locking lids aren’t extravagant extras—they hold the line against moisture and keep the chemical pure.
Even though sulphamidic acid lacks the fireworks of stronger acids, it breaks down under heat and throws off harmful fumes. One summer, I watched a pallet near the hot side of a poorly ventilated room start gassing out. Nobody nearby was prepared for a sudden burst of irritant vapors. Lesson learned. A cool, dry storage area isn’t just theory: it cuts the risk of breakdown and surprise exposure. I never saw a supervisor regret investing in temperature controls and proper ventilation. In fact, the cost of ventilation pays itself back one hundred times over in avoided medical expenses and product loss.
Maybe it sounds obvious, but sulphamidic acid won’t play nice with strong alkalis or oxidizers. I’ve seen the aftermath of one forgotten shelving label, where a small leak dripped onto sodium hypochlorite and filled the air with choking gas. Separate storage for incompatible materials protects more than property—it protects lives. Loud signage, clear barriers, and good training never get old. Day-to-day, I’d rather see redundancies than see someone ignore a faded warning sticker.
Proper labeling stays at the top of my checklist. Nothing causes more confusion than under-marked bins or faded ink. In busy storage areas, fatigue sets in, and mistakes sneak through if people can’t tell what’s in a container at a glance. I always found it helpful to double-check that gloves, goggles, and emergency showers stayed fully stocked and in easy reach of storage sites. Nothing beats the collective confidence of a team who knows the safety gear never runs out.
Sulphamidic acid isn’t going away from industry soon, so the smart move stands with tight lids, cool and dry rooms, clear signage, and ongoing training. The investments in air circulation and hazard communication do more good than fancy technical upgrades. Places that foster a culture of checking, double-checking, and looking out for co-workers rarely struggle with chemical mishaps. In the end, comfort comes from good habits and strong habits start with a team who takes the risks seriously every day.
Sulphamidic acid, also known as sulfamic acid, often goes unnoticed outside labs and industrial workplaces. Once you see its crystalline, powdery form, you realize it’s a lot different from the acids you hear about in high school. The powder usually forms small, colorless, odorless crystals—a sign of its purity, and a reason it’s popular for getting rid of stubborn mineral scale at home and in factories. With a melting point that hovers around 205°C, the stuff holds up well under tough conditions until it finally breaks down.
You’d expect an acid to dissolve well in water, but sulphamidic acid does this with surprising speed, making it handy for cleaners and descalers. One scoop of the powder added to a bucket of water, and you can watch it disappear before your eyes—almost no stirring needed. This quick dissolving power means it works well in quick cleanups, even in cold water, and helps professionals knock out limescale on contact without waiting around. Its solubility also keeps it from leaving behind gritty residue, setting it apart from many other cleaning agents.
Pouring a bag of sulphamidic acid feels different from scooping salt or sugar. The crystals are denser and have an almost sandy flow. Bulk density sits between 2.15–2.16 g/cm³, so a little pile weighs a lot. The grains pack tightly in storage bins, and that heft is helpful in manufacturing and warehouse work, since small amounts stretch further. Still, powder easily creates airborne dust if you pour it too fast, which can irritate the eyes and lungs. Wearing gloves and a mask becomes common sense, not just lab procedure, to avoid unnecessary risk.
One reason crews love working with sulphamidic acid is its stubborn stability at room temperature. You don’t see it catching fire or breaking down under usual conditions. That makes it safe on the shelf, where it sits quietly until needed. Only direct contact with strong bases or substances like chlorine bleach kicks off serious reactions—producing gas that no one wants in a small room. Good ventilation in mixing areas, and never combining it with unknown chemicals, becomes almost second nature among experienced users. This kind of predictability builds trust in the product and keeps accident rates low in daily work.
It’s one thing to read about melting points, but toting a 25-kilo sack through a steamy boiler room shows its real value. Unlike liquids that slosh and spill, the powder coats the inside of kettles, coffee machines, and pipes, delivering direct contact. Cafeterias, power stations, and hotels keep sulphamidic acid in stock for its straightforward action—no frills, no stains, just reliable cleaning. Unopened, tightly sealed containers can sit on a shelf for years without losing punch. This shelf-stable nature lowers waste, cuts costs, and keeps chemical hazards in check in busy facilities.
Pushing for proper usage matters. Anyone handling sulphamidic acid should treat it with the right respect: gloves, eye protection, and instructions printed in plain language on every label. Strong guidelines at workplaces, and honest training, cut down exposure and health claims. Simple fixes—right containers, controlled dispensing, and locked storage—help keep this valuable acid working for the good, not by accident. Real progress in safety comes from listening to people who use these chemicals every day and giving them a say in how safety policies are shaped.
Sulphamidic acid, also called sulfamic acid, shows up in cleaning products, descaling agents, and some industrial processes. Many homeowners unknowingly use it to clean bathroom tiles or remove lime scale from kettles and boilers. Factories turn to it for cleaning metal surfaces, paper bleaching, and even as a food additive regulator. At a glance, this colorless, odorless solid might look harmless, but risks can sneak up where people expect them least.
Anyone pouring leftover cleaning solutions down the drain sends chemicals through wastewater pipes. Most cities trust wastewater treatment facilities to filter out nastier substances before returning the water to rivers or lakes. Sulphamidic acid dissolves completely in water and doesn’t stick to soil or sand. Because of that, it travels fast, mixing wherever the water flows. During my time volunteering at a local river cleanup, we got talking about how many things washing down the sink quietly end up out here. Traces of household acids can interact with metals and other pollutants, neither neutralizing nor staying put.
Acids have a knack for upsetting the delicate balance of streams and lakes. Fish and aquatic insects need a specific pH range to breathe, grow, and reproduce. Pour enough acid into a body of water and it tilts the scales. Some bugs die off, water plants bleach and wither, algae blooms take over, and certain fish—trout, salmon—struggle or vanish. Even small amounts of sulphamidic acid, especially when combined with other household chemicals, slowly add to these changes. Scientists at the EPA documented several stretches of streams in the Midwest where runoff from cleaning solutions had altered the pH and reduced local biodiversity. Invisible tipping points matter.
Sulphamidic acid moves more easily through water than soil. It rarely hangs around long in dirt, as microbes break it into ammonia, sulfate, and water. In the air, traces can react quickly with other compounds, but it hardly ever becomes airborne in the first place. This means the acid’s biggest potential harm sits in wet places, not the air or open fields. On the plus side, earthworms and backyard gardeners likely won’t cross paths with it unless a spill happens near a compost heap or garden bed.
People play a big role. Responsible companies treat wastewater before discharging it. Cities enforce standards for industrial dumping. Curious kids reading product labels at home start to ask parents about safe disposal. Simple steps—running extra water, taking leftovers to hazardous waste drop-offs, choosing the right cleaning strength—keep small slips from stacking up. The more folks know about what escapes down a plughole, the fewer surprises turn up downstream. Schools and community groups host training about safe chemical handling. Even local governments chip in, supporting research on alternative cleaning solutions that fade away without fuss.
Replacing harsh acids with plant-based cleaners appeals to many families. Some factories invest in closed loop systems, recycling water and collecting chemical residues instead of letting anything drift away. Science keeps moving, pushing for safer, greener substitutes in industry and households. Regulations like Europe’s REACH program or America’s Toxic Substances Control Act remind companies and shoppers alike that every chemical choice counts, no matter how invisible it starts out. With a little care, risks shrink and water keeps its life—so does the world outside our doorsteps.
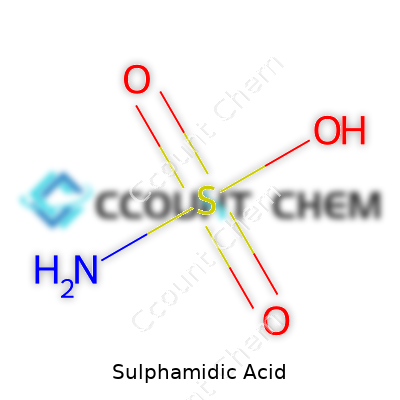
| Names | |
| Preferred IUPAC name | sulfamoylamide |
| Other names |
Sulfamic Acid Amidosulfonic Acid Aminosulfonic Acid Sulphamidic Acid Sulphamic Acid |
| Pronunciation | /ˌsʌlfəˈmɪdɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 5329-14-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Sulphamidic Acid**: ``` Sulphamidic Acid: NH2SO3H JSmol string: -O=S(=O)(N)O ``` **JSmol/SMILES string:** `NS(=O)(=O)O` |
| Beilstein Reference | 1209222 |
| ChEBI | CHEBI:29951 |
| ChEMBL | CHEMBL1587 |
| ChemSpider | 8470 |
| DrugBank | DB01591 |
| ECHA InfoCard | ECHA InfoCard: 100.005.777 |
| EC Number | 226-218-8 |
| Gmelin Reference | 77085 |
| KEGG | C06191 |
| MeSH | D013430 |
| PubChem CID | 542S |
| RTECS number | WO9700000 |
| UNII | LYC56JV361 |
| UN number | UN2967 |
| Properties | |
| Chemical formula | H3NSO3 |
| Molar mass | 97.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.126 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.13 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.1 |
| Basicity (pKb) | 11.18 |
| Magnetic susceptibility (χ) | -49.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.601 |
| Dipole moment | 2.18 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 96.3 J K⁻¹ mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -325.8 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1035 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | A09AB07 |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage. Harmful if swallowed. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H318 |
| Precautionary statements | H302, H314, P260, P264, P270, P280, P301+P312, P301+P330+P331, P305+P351+P338, P310, P321, P330, P363, P405, P501 |
| NFPA 704 (fire diamond) | 2-0-0-Acidos |
| Lethal dose or concentration | LD₅₀ (oral, rat): 3160 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3160 mg/kg (oral, rat) |
| NIOSH | RT8750000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sulphamidic Acid: 10 mg/m³ |
| REL (Recommended) | REL: 0.5 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Sulfanilamide Sulfamic acid potassium salt Sulfanilic acid Sulfuric acid Amidosulfonic acid |