Industrial chemistry changed a lot after researchers brought sulfamic acid into the public eye in the late nineteenth century. European chemists started working out ways to combine urea with fuming sulfuric acid, opening up a reliable route to a stable, crystalline compound in 1834. Over time, manufacturers caught on to its utility thanks to its role in descaling and household cleaning. By the 1920s and 1930s, several patent races began in earnest, mostly between German and American companies, who saw that sulfamic acid could replace more volatile and inconvenient acids for routine tasks. If you worked in a paper mill, a sugar refinery, or a chemical plant in those days, you started to see bags of sulfamic acid take over from harsher, less predictable acids like hydrochloric or nitric. It wasn't as explosive, didn't leave behind fumes, and the leftovers could be safely disposed with less environmental impact. Generations of chemists and plant managers leaned on these advantages to streamline their processes.
Sulfamic acid shows up on the shelf as a white, odorless powder. People have lumped it under several names, including amidosulfonic acid, amidosulfuric acid, aminotrisulfonic acid, and sulphamidic acid. Each one points back to the chemical formula, H3NSO3. Whether buying it in kilo bags or tonnage quantities for industrial purposes, the chemistry stays the same. The dry solid stores well and dissolves easily in water, forming clear, acidic solutions. If you've worked in water treatment, you've seen it on the dosing tables right alongside the classics like citric and acetic.
In the lab or on a warehouse floor, sulfamic acid acts both predictably and robustly. Its melting point floats around 205°C, but before you get there, decomposition sets in, releasing sulfur oxides and ammonia. You won't see fumes under dry, moderate storage. Solubility in water runs high—just pour in a spill or make up a concentrated cleaning solution and there's no trouble mixing. Once dissolved, the solution measures close to a pH of 1, hanging tight with other strong acids. It doesn’t attack most plastics, but it eats through carbon steel and aluminum, so always use rubber-lined gear or stainless when prepping or storing solutions. Most folks find its stability and non-hygroscopic nature a real benefit; it won’t clump or degrade on the shelf, even in humid climates.
Most standards put minimum purity near or above 99.5%, with small traces of moisture allowed as long as they don’t impact functionality. Industrial suppliers often provide detailed certificates of analysis: sulfur trioxide content, ammonia, iron, chloride, and heavy metals. Labels signal corrosive risk, with clear hazard pictograms for eye and skin damage. GHS and DOT rules require tightly sealed, high-density polyethylene drums with hazard warnings in local languages and English. In my time working logistics at a chemical distributor, enforcement varied country to country, but proper labeling and documentation made all the difference in customs or safety inspections.
The main way to get pure sulfamic acid goes back to reacting urea, the same stuff found in fertilizer, with fuming sulfuric acid or chlorosulfonic acid. This pathway brings in concentrated reagents and requires a controlled exothermic reaction, usually in a stirred glass-lined reactor under strong ventilation to trap off-gasses. The crystallized product gets filtered off, washed, and dried under vacuum. Chemists sometimes employ batch methods for kilo lab quantities, but continuous-flow reactors handle the modern ton-scale output. You can easily find detailed reaction schemes in synthetic organic chemistry manuals. The biggest risks lie in exothermic surges and handling strong mineral acids—anyone familiar with plant accidents knows the importance of staying on top of temperature and pressure controls during this prep.
In the hands of a skilled technician, sulfamic acid performs well in both industrial and analytical reactions. It hydrolyzes slowly with water, yielding ammonium bisulfate and then sulfuric acid as the reaction runs to completion, especially if heated or exposed to bases. Strong oxidizers convert it to nitrogen and sulfate. Mixed with nitrites, it can produce nitrogen gas—a trick used in analytical methods to destroy excess nitrite before measuring ammonia in water samples. Chemists also use it as a milder sulfonating agent for making dyes or pharmaceutical intermediates. You’ve probably seen it as a descaling acid; it chews up calcium and magnesium deposits without biting into stable alloys or rubber. Subtle modifications to the molecule—adding certain organic groups—produce specialty products for fire retardant agents and pulping aids.
Sulfamic acid’s exact name depends on where you see it. Across North America and Europe, “sulfamic acid” leads the pack, but labels sometimes read “sulphamidic acid” or “amidosulfonic acid” in older texts and global trade shipments. Big manufacturers like BASF, Merck, and Sigma-Aldrich each market their own “brand” grades, often differentiated by particle size, purity, packaging, or targeted applications. Whether the bag features chemical symbols or only a product code, read the technical data sheet before making a purchase—different applications call for different trace impurity thresholds, especially in food or medical settings.
Handling sulfamic acid requires proper respect. The dust can irritate or burn respiratory passages with a big enough exposure, and wet solutions burn skin or eyes as fast as any strong acid. Workers should suit up with goggles, gloves, face shields, and acid-resistant aprons. Storage asks for a dry, cool spot out of reach of bases or hypochlorites—mixing mistakes generate chlorine gas or corrosive vapors. Emergency kits at my workplace always included plenty of running water, neutralizing agents, and first-aid for acid splashes. Big industrial users go a step further, using fixed air ventilation, spill capture, and continuous pH monitoring at juncture points to catch leaks or accidents before they spread.
Sulfamic acid reaches into cleaning, descaling, pulp and paper, water treatment, and the food industry. Home users find it as a main ingredient in kettle and coffee maker descalers; industrial users rely on it for cleaning boilers, heat exchangers, and piping systems fouled by mineral scale. In pulp mills, it helps break down lignin during pulping, allowing for easier paper processing. Textile and dye companies benefit from its seat as a sulfonation intermediate. Pool maintenance professionals count on it for pH correction that won’t introduce harsh byproducts. Even the food industry relies on highly purified forms for cleaning-in-place routines or direct food contact cleaning where residue regulations run tight. Labs everywhere keep it for stripping nitrite or cleaning glassware without the lingering fumes of hydrochloric acid.
Current work on sulfamic acid targets both greener production methods and expanded utility. Researchers focus on synthesizing it via less energy-intensive routes, using milder solvents, or integrating waste heat from other reactions—driven by regulatory crackdowns on emissions and carbon footprints. Chemists and chemical engineers pull data from environmental monitoring to predict downstream effects and tweak processes accordingly. On the product performance side, R&D explores specialized composite formulations for targeted cleaning and water system recovery. Applications in catalyst development and pharmaceutical manufacture keep growing, with tweaks to sulfamic acid’s structure unlocking new ways to bind or activate substrates. From my own experience in lab-scale trials, minor changes to reaction pH, concentration, or sequencing prove critical in balancing yield, purity, and operational risk.
Human health studies measured toxicity carefully over the past fifty years. Most toxicology data points to low acute oral and dermal toxicity, though the acid’s corrosive nature drives local tissue damage rather than systemic poisoning. Inhalation of swirling dusts doesn’t lead to chronic toxicity, but if you’ve breathed the stuff deeply, you know the pain of inflamed sinuses and lungs. Chronic exposure in poorly ventilated plants led regulators to demand strict occupational exposure limits. Wastewaters bear watching—local acidification can disrupt aquatic life and downstream processes, so environmental controls guide discharge tightly. Extensive animal studies and regulatory reviews across continents shaped the labeling and safe handling procedures now found in every facility manual.
Sulfamic acid likely stands as one of the most durable actors in the chemical toolkit for decades to come. Process industries keep discovering fresh niches—from microelectronics etching to biotechnology platform cleaning. R&D keeps pushing out incremental advances in both sustainability and specialty markets. More countries will likely regulate and recycle water and wastes from cleaning processes, fueling the search for ultra-low residue products and tailored blends. The combination of strong acid power with manageable safety and waste impact means newer industries eye it as a bridge to cleaner and more circular process cycles. Personal care, battery recycling, and environmental remediation might all see growing sulfamic acid use as technical needs shift and regulatory climates evolve. Based on its performance and ease of use, it’ll keep drawing interest in research labs, production facilities, and even home applications for years ahead.
Sulfamic acid rolls off the tongue a bit awkwardly, but if you've ever descaled a kettle or watched a tile floor regain its shine, this compound probably deserves some credit. In my years cleaning up stubborn mineral stains in my own kitchen, the power of strong acids has been obvious—sulfamic acid belongs on that list, packing enough punch to tackle calcium deposits without the edge that something like hydrochloric acid brings.
Household cleaning products lean on sulfamic acid because it dissolves limescale that builds up inside kettles, dishwashers, coffee machines, and bathrooms. Tap water leaves behind mineral traces, and basic soap scum rides along with it. Sulfamic acid cuts through both. Compare it to vinegar—the old school trick for coffee machines—and it’s easy to see how much faster and more thoroughly these commercial descalers work. That’s why hotels and restaurants use it: large-scale cleaning done right keeps customers safe and appliances working.
Industrial operations face the same fight against scale and residue, but on a much larger scale. I’ve walked through factories that can’t afford equipment to go down just because of unwanted buildup. Water-cooling towers and boilers clog up quickly. Sulfamic acid keeps the pipes open, the heat exchangers working, and the water flowing. Unlike some harsher acids, sulfamic acid won’t start corroding the metal surfaces underneath, so it doesn’t turn a cleaning job into a repair bill.
In swimming pools, chlorine tablets sometimes use sulfamic acid to stabilize the mix and keep things sanitary without a chemical rollercoaster. Pool owners know that clean water isn’t a given—it’s a result of steady chemistry and some vigilance. Chemically balancing water is all about removing what doesn’t belong, and preventing stains or algae growth. Sulfamic acid isn’t just a cleaner. It’s a tool for building trust in public spaces where water hygiene protects health.
Sulfamic acid also plays a behind-the-scenes role in paper and textile manufacturing. Dyeing and bleaching need reliable, non-volatile acids. Industries pick sulfamic acid because it’s less hazardous than sulfuric or hydrochloric acid, but still gets the job done. The acid helps fix colors in textiles and removes the brown tint from pulped wood, setting the stage for newspaper print and crisp white fabrics. It isn’t glamorous work, but it matters every single day.
Accidental exposure to strong acids isn’t something to take lightly. Google and several safety bodies list proper protective gear as standard for anyone handling concentrated sulfamic acid. Burns, eye damage, and respiratory problems are all real risks with improper use. I’ve always kept gloves handy, and the lessons from a single splash keep safety top of mind. Clear labeling and common sense mean fewer accidents—a simple way to make workplaces and homes safer.
Eco-friendly cleaning has turned into more than a trend; people want products that work well and don’t poison waterways or bleach out garden soil. Sulfamic acid, when handled and disposed of properly, has a smaller environmental footprint than other strong acids. It breaks down to harmless components and poses less threat to wildlife down the drain. Reading product labels and following use directions does more than just keep your appliances clean; it keeps what's downstream just a bit safer too.
Sulfamic acid pops up in laundry rooms, pools, and even in factories. You might see it on a bottle of descaler you use for kettles, or pool chemicals at the hardware store. Its main job is to dissolve lime scale, rust, and mineral buildup. People like it over harsh stuff like hydrochloric acid because it seems more manageable, doesn’t fume, and won’t eat away at your pipes if you treat it right.
Every acid brings a risk, even the stuff labeled “safe for home use.” Sulfamic acid comes as a white powder or granules. Touching it can sting your skin, especially if you don’t rinse it off fast. Breathe the dust, and your throat can burn. Let a bit splash into your eye, and you’re heading to the emergency room.
I remember unclogging a coffee machine boiler with it. I wore gloves, but distracted, I knocked over the cup with the solution. The splash seemed minor at first. After a few minutes, my hand felt like it was on fire. Nothing serious happened because I flushed it quickly, but it hammered home how even “safe” cleaners come with a catch.
Mixing chemicals always spells danger. Sulfamic acid and bleach do not play nice together. The combination spits out toxic gases, including chlorine, which can send someone to the hospital after a single breath. In kitchens, restaurants, and cleaning gigs, cooks or janitors sometimes grab whatever works fastest, thinking stronger solutions mean faster results. Rushing leads to mixing, and mixing leads to chemical clouds nobody wants.
Dilution mistakes are common. Too strong a solution, and the acid can eat through metals, not just the gunk you’re trying to remove. Overused on chrome or decorative fixtures, it leaves dull, pitted spots. Pouring unused acid down the drain also causes problems for your local water treatment plant and aquatic life.
Researchers rate sulfamic acid as less toxic than many harsh cleaners. The U.S. Environmental Protection Agency says that with everyday cleaning, it doesn’t build up in your body or linger long in the environment. Respiratory exposure stays low unless you handle big amounts or work in a tight space. Skin exposure often causes only irritation, not dangerous burns, unless the solution is highly concentrated.
Safety data sheets agree: use gloves, splash-proof goggles, and keep workspaces vented. In Europe, regulators classify it as not carcinogenic, not likely to damage fertility, and unlikely to harm kids from secondary contact. You should still treat it with the same respect given to all acids.
To use sulfamic acid without trouble, a few habits help. Label spray bottles and don’t transfer chemicals to old drink bottles. Always wear gloves. Eye protection saves the day in close cleaning jobs, especially around faucets or overhead fixtures. Never mix it with products containing bleach or ammonia. Store it in a cool, dry place, out of kids’ reach.
For the environment, avoid tossing leftovers into sinks. Neutralize it with a base like baking soda, then dispose according to local rules. If your business uses a lot, regular safety training and clear instructions keep surprises to a minimum.
People keep looking for safer cleaning products. Some eco-friendly options work for mild cleaning, but nothing cuts through thick scale quite like acid. Better labeling and staff education could lower accidental exposures, especially in busy settings. Wider use of personal protective equipment remains a practical answer, not just a legal requirement.
From home fix-ups to pool servicing, respect for chemicals goes a long way. Knowing what’s on your hands—and in the air—keeps both your skin and lungs in better shape.
A bag of sulfamic acid doesn’t look like much, but there’s a lot more going on than meets the eye. Years ago, during my time in an industrial setting, I saw firsthand how mishandling this chemical can disrupt operations and cause injuries. This material might get tossed around carelessly, but it demands respect. Sulfamic acid reacts with water, releases heat, and if stored near incompatible substances, does more harm than good. So, the goal remains clear: protect workers, equipment, and the environment by paying close attention to storage habits.
Many folks in maintenance and cleaning overlook the basics. I remember a time when unopened bags got stacked right next to mop buckets and bottles of bleach — a near disaster. Sulfamic acid reacts violently with chlorine-based products. Facilities should dedicate a well-marked, dry, and cool room or cabinet for storage. Humidity shouldn’t creep in, and sunshine doesn’t do any favors. Exposure to moisture not only encourages clumping but creates a chance for acid vapors to form. Nobody wants to breathe that in, and once the product starts breaking down, strength drops, and results become unpredictable.
No one ever forgets the stench after an accidental mix with bleach, but more often than not, trouble comes from storing incompatible substances together. Sulfamic acid belongs nowhere near oxidizers, strong bases, or metals like aluminum and zinc. Even among acids, it prefers its own company. Plastic containers with tight-fitting lids do the trick; metal or breakable glass can lead to leaks and headaches. Keep the original packaging intact, and always check for cracks or signs of wear. After years of handling chemicals, I know corroded shelves or containers cause more emergency calls than most people realize.
Some of the best safety improvements start with a simple lock and a clear sign. Unauthorized staff, especially those less experienced, shouldn’t have easy access. Training goes hand-in-hand with storage — every person near the storage area should know what sulfamic acid looks and smells like, what not to mix, and how to handle spills. I still carry the memory of a rushed janitor pouring powder into standing water. He didn’t mean harm, but ignorance nearly put him in the hospital.
A responsible workplace keeps spill kits, respirators, and goggles close to the door. Acid-resistant gloves aren’t optional, and emergency eyewash stations prove their worth after one panicked worker runs for help. In my experience, even those who work with harsh chemicals daily skip gloves, only to pay for it with red, irritated skin. Training saves skin and lives. Every year, the American Association of Poison Control Centers gets reports on accidental exposures. Most accidents stem from poor planning, not bad luck.
Don’t stash barrels and forget about them. Assign someone to do monthly checks. Keep records. If a bag feels damp or starts falling apart, arrange for hazardous waste pickup. Never dump leftovers down a drain — water authorities track these incidents, and fines show up fast. Waste handlers know what to do with expired or compromised product, so let professionals step in.
Safe storage of sulfamic acid doesn’t require a chemistry degree, but it does demand commitment and a bit of common sense. Sticking to tried-and-true practices — dry storage, physical separation, careful labeling, and plenty of education — lays the groundwork for a safer environment, inside and outside the workplace. The details matter. Small steps today keep people out of trouble tomorrow.
Sulfamic acid helps with descaling, cleaning, even pulp and paper bleaching. This is all useful—until someone spills the bag or breathes in the dust. My own time working at a small metal plating shop taught me the difference between care and disaster. We had one fellow who bragged how nothing fazed him. He didn’t wear gloves. Stripped the thickest rust for years, hands bare, till his skin looked leathery and red. The lesson stuck: taking short-cuts with chemicals feels easier, but it bites back.
Breathing in sulfamic acid dust can hit your lungs hard. It can trigger coughing, sore throat, shortness of breath. Splashes burn your eyes before you even blink. Spilled powder on skin means itching, blisters, or worse. A scoop in the wrong place starts odd-smelling fumes, sometimes enough to clear the room.
Official warnings ask us to treat this stuff with respect. According to the Centers for Disease Control, direct contact or inhalation can cause severe irritation and burns. Even small doses matter. The European Chemicals Agency lists it as hazardous for skin and eyes, and as an environmental pollutant, so it shouldn’t end up down the drain.
Gloves and goggles really do work. Nitrile or neoprene gloves hold up much better than latex when handling acids. Safety glasses with side shields, plus a dust mask or a full-face shield if you’re mixing more than a scoop. If the acid is in solution, a rubber apron or lab coat keeps your shirt and skin clean.
Wear closed shoes because a dropped scoop finds your toes fast. In real workplaces, folks try to finish the job quickly, but these basic protections slow down injuries, not the workflow.
Only mix sulfamic acid in well-ventilated spaces, preferably under an extractor fan. Even in a home garage, an open window and a standing fan aimed at the fumes makes a difference. Never add it to hot water, since that releases vapors fast and increases the risk of a spill.
Store bags or containers high and dry, locked away from curious hands and food supplies. Label everything in big, bold letters. Accidental swaps lead to kitchen disasters. I’ve seen a janitor mistakenly use acid instead of salt and ruin a cafeteria sink—and nearly his own hands.
Keep a jug of water and an eye-wash bottle nearby for emergencies. Baking soda neutralizes acid in case of a small spill, but don’t dump it in all at once, or you’ll get a bubbling mess. Clean up with plenty of water, never with ammonia or bleach mixtures, since that can create dangerous fumes.
No one gets it perfect every time, but working with chemicals like sulfamic acid teaches attention to detail. The stories of burns and close calls aren’t just old shop tales—they’re reminders that these hazards are real. By respecting warnings, using proper gear, and staying alert, the chances of injury or error drop sharply. In the end, thoughtful handling keeps everyone safe, from the most seasoned worker to the new apprentice sweeping the floor.
Cleaning products promise miracles, but mix the wrong chemicals, and you’ve got danger brewing in your mop bucket. Sulfamic acid—a favorite for removing limescale and heavy mineral deposits—works wonders on its own. In my professional cleaning days, I saw what happened when folks tried to make homemade “super cleaners” by combining acids and off-the-shelf products. Bathrooms smelled like science experiments gone wrong, and worse, I heard of cases where someone wound up at the doctor with burning eyes or lungs.
Sulfamic acid stands out because it’s strong enough to break down stubborn mineral scale but safe enough to handle, if you follow instructions. You’ll find it in descalers and some toilet bowl cleaners. It dissolves hard water stains, and leaves metals and ceramics shining. The trouble starts when you think adding more active ingredients brings extra cleaning punch.
Acid reacts with other chemicals fast and unpredictably. Blend sulfamic acid with bleach, for example, and you risk releasing toxic chlorine gas. Even household ammonia—harmless on its own—becomes hazardous when mixed with acids. Years ago, I watched an experienced janitor accidentally inhale fumes from an acid and bleach mix. He spent the afternoon with the nurse, eyes streaming and coughing. That left a mark on everyone there: some cleaners just don’t belong together.
Manufacturers run tests to make sure chemical mixtures don’t start fires, corrode pipes, or harm users. Once you add something not listed on the label, no one can promise how those solutions will react together—or in your drains, on your hands, or in the air you breathe. For this reason, consumer safety groups and building maintenance experts agree: Keep sulfamic acid apart from other active cleaners.
Labels on cleaning products tell a clear story. If sulfamic acid is your go-to, use it for its intended job. Rinse the surface well before introducing anything new. Resist the urge to “boost” cleaning action—deeper stains or deposits often need time, not stronger cocktails.
I learned from real-life messes, not just textbooks. It’s easy to lose patience, want faster results, and grab the nearest second product, hoping to shave minutes off the job. That shortcut leads to damaged surfaces, ruined safety gear, or a call to poison control. Many trade professionals keep the number for the local hazardous materials response team handy—not because they’re reckless, but because just one mistake with mixtures can turn routine work into an emergency.
Education does more than a warning label. Schools and job sites benefit from regular training on which chemicals mix safely and which don’t. Online resources provided by government agencies and reputable health organizations stay updated with the latest accident reports and recommendations. I tell friends and coworkers: stick to one product at a time, follow the instructions, and wear gloves and proper eye protection. Cleanup becomes less of a gamble.
Cleaning should solve problems, not create new ones. Sulfamic acid still deserves its place on the shelf—but only on its own. Give each chemical its space, and you’ll keep both your results and your well-being in good shape.

| Names | |
| Preferred IUPAC name | Sulfamic acid |
| Other names |
Aminosulfonic acid Aminosulfuric acid Amidosulfonic acid Sulfamidic acid Aminosulphuric acid Sulphamic acid |
| Pronunciation | /ˌsʌlˈfæmɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 5329-14-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Sulfamic Acid**: ``` NH2SO3H ``` This is the SMILES string: ``` NS(=O)(=O)O ``` |
| Beilstein Reference | 1718735 |
| ChEBI | CHEBI:29939 |
| ChEMBL | CHEMBL1239 |
| ChemSpider | 5376 |
| DrugBank | DB09312 |
| ECHA InfoCard | 100.008.954 |
| EC Number | 226-218-8 |
| Gmelin Reference | 849 |
| KEGG | C00284 |
| MeSH | D013212 |
| PubChem CID | 6112 |
| RTECS number | WO6125000 |
| UNII | U054VJO880 |
| UN number | 2967 |
| Properties | |
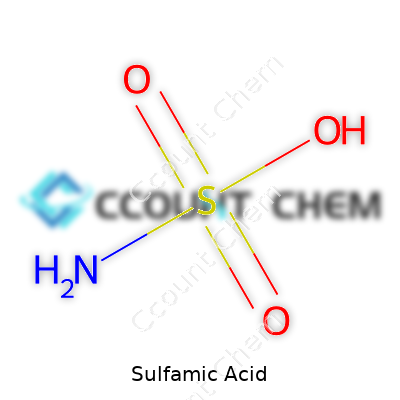
| Chemical formula | H3NSO3 |
| Molar mass | 97.09 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 2.126 g/cm³ |
| Solubility in water | Freely soluble |
| log P | -4.34 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 0.99 |
| Basicity (pKb) | 0.35 |
| Magnetic susceptibility (χ) | -43.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Dipole moment | 1.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 82.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −531.4 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -532 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | V03AX19 |
| Hazards | |
| Main hazards | Corrosive, causes burns to skin and eyes, harmful if swallowed, emits toxic fumes when heated, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | DANGER |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P312, P405, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 oral rat 3160 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3160 mg/kg (Rat, oral) |
| NIOSH | KWVS0007 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Sulfamic Acid: "15 mg/m³ (total dust), 5 mg/m³ (respirable fraction) as nuisance particulate |
| REL (Recommended) | 30 mg/m³ |
| Related compounds | |
| Related compounds |
Amidosulfonic acid Sulphamide Sulfamide Sulfanilamide Sulfanilic acid |