Some chemical compounds stay behind the curtain, quietly making modern life tick. Sodium xylene sulfonate, a surfactant and solubilizer, fits that mold. Chemists began producing alkyl aryl sulfonates in the early twentieth century as the demand for synthetic detergents grew fast alongside indoor plumbing and a desire for better cleaning products. By mid-century, sulfonation of xylene led to the creation of sodium xylene sulfonate—lighter on the environment than many earlier laundry agents. Over the decades, researchers tweaked production for higher yield and lower cost, reflecting a broader trend toward safer and more environmentally responsible manufacturing in the chemicals industry. That ongoing push for greener chemistry shaped the modern approach to making and using sodium xylene sulfonate today.
Decades of daily chemistry turn this compound into an easy-to-handle solid or clear liquid, depending on concentration and formulation. Its biggest job involves breaking up oils and dirt and helping other ingredients dissolve in water—without fragrance or heavy color to spoil the rest of a formula. Cleaning products for households and industry, shampoos, skin cleansers, even paints rely on its tough but gentle character. I remember mixing a batch of liquid soap in a small company, seeing sodium xylene sulfonate bridge the divide between cloudy chaos and a clear, stable blend.
Anyone who opens a drum of sodium xylene sulfonate notices its strong chemical odor and fine white crystal or powder, though the liquid form is nearly odorless and looks like water. It dissolves rapidly in water, holding up from neutral to basic pH, and tolerates high and low temperatures alike. The molecular structure—a sodium salt of sulfonated methyl-benzenes—contributes to its excellent solubility and ability to cut through oils. Unlike harsher surfactants, it stays relatively mild on skin, an important factor in the push for gentler personal care products.
A proper technical spec sheet for sodium xylene sulfonate runs long. Key purities range from 40-80% in aqueous solutions, with low levels of unwanted organics and heavy metals. Reliable producers offer regular certificates of analysis, batch tracing, and proper labeling. Toxicological information, composition, recommended storage, and transport advice fill out each safety data sheet. Regulatory codes from REACH and other agencies must appear, alerting handlers to precautions and potential risks in a direct, understandable way.
Manufacturers synthesize sodium xylene sulfonate by sulfonating xylene with sulfuric acid, cooling it, and neutralizing the resulting xylene sulfonic acid with sodium hydroxide. The process generates a sodium salt with mostly the ortho and para isomers predominating. After neutralization, removal of excess acid, and purification, the product enters industrial drums or holding tanks before shipment. I've seen operators watching temperature curves closely, as over-sulfonation can waste feedstock and produce unwanted byproducts.
In the lab, sodium xylene sulfonate holds up across a wide pH and temperature range. It resists most chemical attack under normal handling, but does react with strong acids or oxidizers, breaking down to simple aromatic sulfonates and sometimes to lesser-known combustion products. Researchers searching for milder, more biodegradable surfactants occasionally start with sodium xylene sulfonate, adding hydrophilic groups or tweaking the xylene base to tailor performance. Its backbone supports further chemical modification without collapsing the surfactant function.
In the tangled world of chemical commerce, sodium xylene sulfonate appears under multiple names. Some call it sodium toluene sulfonate, SXS, sodium dimethylbenzene sulfonate, or simply xylene sulfonate. Multinational firms register trademarked blends built around this key ingredient, but the underlying chemistry remains consistent. Familiarity with this spectrum of synonyms matters, especially when reviewing international shipments, safety information, or emerging research.
The risk profile for sodium xylene sulfonate runs low for most practical uses, yet proper ventilation and minimal direct contact with eyes or skin matter in handling. Dust masks help prevent respiratory discomfort when mixing or transferring the fine powder. Contact can dry or irritate skin after repeated exposure, but compared to other industrial surfactants, sodium xylene sulfonate ranks as milder. Emergency eye-wash stations and gloves form part of good standard operating procedure. Environmental regulators in Europe and North America classify it as biodegradable with low aquatic toxicity, a point worth noting for any firm concerned about wastewater compliance.
The list of uses stretches from home to heavy industry. Most people encounter sodium xylene sulfonate in shampoos, hand soaps, laundry detergents, and household cleaners. Its ability to boost the solubility of other ingredients without wrecking skin or surface finishes gives formulators broad room for creativity. Metal cleaners, degreasers, oilfield chemicals, textiles, and agricultural sprays take advantage of these same traits. Paints and coatings rely on it for flow and pigment stabilization. Having consulted on cleaning formulations, I’ve seen how a small dose of sodium xylene sulfonate solves solubility bottlenecks, making a formula both affordable and tough on grime.
The wave of research into greener surfactants affects sodium xylene sulfonate much like it does every major cleaning chemical. Lab work aims at two improvement tracks: greater biodegradability and higher function under tough water conditions. Blending it with plant-based surfactants or designing it to break down faster in municipal wastewater systems can reduce environmental loads. Scientists study how its molecular tweaks influence foam generation, grease removal, and compatibility with sensitive skin—crucial questions as demands grow for milder yet more effective cleaning agents.
Across studies, sodium xylene sulfonate demonstrates low toxicity towards aquatic and terrestrial life, particularly compared to harsher detergents. Standard toxicity testing shows a high lethal dose, pointing to minimal risk in household exposure. Still, emerging research watches for chronic effects or subtle disruptions to aquatic food webs, especially as cumulative exposure grows. Manufacturers continue to publish new research on degradation byproducts, making sure that commercial use doesn’t leave behind hidden risks. Safety committees in Europe and North America call for ongoing surveillance and data sharing from producers, a way to update protocols and refine chemical handling advice.
While some push toward ultra-green chemistry may one day nudge sodium xylene sulfonate out of common use, for now, it bridges the gap between performance and safety better than many rivals. Refinements in production offer reduced energy input and less toxic effluent. Advanced chemical engineering could make xylene sources less dependent on fossil fuels, supporting a broader shift toward sustainability. Collaboration between chemists, health experts, and regulators will shape its next act—fewer environmental burdens, greater biodegradability, and transparency across the supply chain. As cleaning challenges evolve, sodium xylene sulfonate should keep adapting—steady workhorse in a world demanding reliable, safe, and affordable solutions.
Most people don’t read the label on their bottle of dish soap or floor cleaner. Even if you do, a name like sodium xylene sulfonate sounds like something that belongs in a chemistry lab. The truth is, this oddly named ingredient shows up in more corners of daily life than most realize. So why? It’s all about how it breaks down dirt, keeps liquids from separating, and helps products stay affordable.
In my years working in a hardware store, I stocked shelves with dozens of cleaning agents. Clients often asked why their hand soap felt smoother than regular bar soap or why bathroom sprays left no streaks. The answer boiled down to certain chemicals that improve flow, reduce clumping, and boost cleaning strength. Sodium xylene sulfonate crops up here—a workhorse additive for both homes and industry.
This compound acts as a surfactant. In plain talk, it helps water mix with grease or oil, loosening grime that would otherwise stick. Almost every household and industrial cleaner relies on some surfactant, and sodium xylene sulfonate is chosen because it’s cost-effective and works in both hard and soft water. Its popularity comes from its ability to keep products consistent from bottle to bottle.
During college, I did a stint at a skincare company and learned what sets a "gentle" product apart from one that dries you out. A low amount of sodium xylene sulfonate shows up in many facial cleansers and shampoos. Some people worry about chemical additives in products they put on their bodies. I share that concern. Scientific studies suggest this compound doesn’t build up in the body and doesn’t act as an irritant for most people, but it’s always wise to patch test, especially when you have sensitive skin.
Factories use sodium xylene sulfonate to keep their chemicals from clumping or separating. Paints, dyes, and coatings need a stable blend to prevent jams in machinery or uneven coverage. The additive stops thick liquids from gelling up in pipes or tanks. It also helps companies save money because they can use less of expensive ingredients and still get the same slippery, spreadable result.
Many shoppers want biodegradable cleaners, concerned about what washes down the drain and into rivers. Sodium xylene sulfonate breaks down in wastewater treatment plants more readily than some older additives. Even so, eco-conscious brands are trying out plant-based surfactants, especially when there’s growing demand for reduced environmental impact.
Some industry insiders admit sodium xylene sulfonate isn’t perfect for everything. In certain formulas, too much can leave a residue or add an unwanted odor. Chemists are working on blends that perform the same job with fewer downsides. As more people push for company transparency, ingredient lists have become easier to read, helping shoppers make informed choices.
Taking a closer look at sodium xylene sulfonate opens up questions about what goes into everyday items. Knowledge gives customers the choice to find alternatives if they want, and helps companies tweak formulas to match changing values. Trust relies on how well companies and regulators share information about these ingredients—something that’s only become more important over time.
Sodium Xylene Sulfonate shows up on a lot of ingredient labels—cleansers, shampoos, even some hand soaps. Manufacturers like to use it to help mix oil and water, breaking up surface tension so a formula doesn’t separate. It helps create that satisfying lather most of us expect from soaps and washes.
Safety means more than just “not toxic.” It’s about real-life use: how people react after a bottle’s been in the shower for months, or how often kids grab mom’s shampoo. When a manufacturer uses Sodium Xylene Sulfonate, the concentrations usually sit at less than 5%. The Cosmetic Ingredient Review Expert Panel looked into it, noting no evidence suggests major problems in these typical amounts for most people.
Unlike strong detergents or harsh solvents, Sodium Xylene Sulfonate belongs to a group designed for skin and home use. In my own experience with sensitive skin, I’ve watched hundreds of ingredient lists and have tried to connect the dots on reactions. Most reports of skin issues—stinging, redness, flaking—come from fragrances and strong preservatives, not from this sulfonate. Households with eczema or dermatitis sometimes have a harder time, but those cases often come down to other, more aggressive ingredients mixed with it, not the Sodium Xylene Sulfonate itself.
Dermatology studies and toxicology reports give some peace of mind. The US Food and Drug Administration and the European Chemicals Agency have checked Sodium Xylene Sulfonate for skin sensitization and irritation. As long as the percentages stay low, tests show a low chance of causing problems. Not every ingredient gets this kind of research focus, so its track record matters. Still, laboratory tests only go so far. Long-term, widespread use, especially in repeated, high-exposure settings, tells us most of what we need to know.
Stories collected by skin care support groups or shared on medical forums seem to back up the research. Cases of true allergy to Sodium Xylene Sulfonate remain rare. Temporary redness or dryness sometimes happens if people use a cleanser with a bunch of other surfactants, or if someone already deals with skin conditions. Most reactions seem tied to other ingredients or excessive washing, not the sulfonate.
Choosing a gentle routine always makes sense. If you’re already prone to sensitivity, stick with short ingredient lists, fragrance-free products, and formulas designed for delicate skin. Look at what else sits in the bottle before blaming Sodium Xylene Sulfonate. Patch testing can help spot the culprit behind any reaction.
Transparency matters. Brands that open up about their formulas and provide patch test advice empower their customers. Trusted third-party organizations, like the Environmental Working Group or SkinSAFE, publish ingredient safety grades based on the best available science. Checking their resources lowers the risk of running into surprise irritation or allergy.
Personal experience shows people benefit when skin care companies focus on honest communication. Clearer ingredient lists and better education about why something is used and in what concentration can take guesswork out of shopping for new products. Advocacy for research, especially on long-term, layered use of multiple ingredients, will help steer the market toward even safer skin care choices.
Sodium xylene sulfonate (SXS) turns up often in cleaning products and industrial formulations. At its core, it’s a clear, colorless liquid or sometimes a solid, depending on how concentrated it gets. It slips easily into water, melting away with very little effort, which is a key reason chemists reach for it when they want to make stubborn ingredients dissolve better together. If you’ve stood in front of a shelf with soaps or detergents, you’ve probably seen the results of SXS helping make those liquids look clear and pour easily, instead of separating into globs or clumps.
Its chemical formula, C8H9NaO3S, puts it in the family of sulfonate salts. Toss it into water and SXS just disappears—there’s no gritty residue and scraping at the bottom of the tank. It doesn’t have a strong odor, which makes life easier for the people working with large amounts on the factory floor. In most blends, SXS stays stable even when heated up or cooled down, so folks in manufacturing know they won’t have a surprise chemical reaction in switching seasons or varying storage conditions.
This compound doesn’t just dissolve itself; it’s used most often to help other ingredients break up and stay put in water-based mixtures. If you’re dealing with a detergent that thickens up too much, SXS acts as a thinning agent so the mixture flows more like a liquid and less like a paste. It’s one of those behind-the-scenes helpers that makes sure products feel right in your hand or spread properly over a surface.
People working with SXS tend to appreciate that it has a low toxicity profile. According to the U.S. Environmental Protection Agency and the Cosmetic Ingredient Review, this chemical rarely causes skin or eye irritation at the concentrations used in personal care or cleaning products. In my years around both household and shop-grade cleaners, no one ever raised major health flags connected to SXS. Still, like with a lot of salts, swallowing a large amount or direct contact with the eyes would be uncomfortable, and good gloves and goggles never go out of style in production.
SXS doesn’t seem to stick around long in the environment after it’s washed down the drain; microbes and sunlight break it down. I’ve read studies showing limited build-up or harm to aquatic life, so regulatory groups generally allow its use. That being said, it’s always worth double-checking supply chain practices and wastewater treatment, because what looks harmless can stack up if manufacturing corners get cut.
Companies could keep pushing for greener production methods. Sourcing raw materials from renewable resources instead of petroleum makes good sense. More frequent testing of batches can catch impurities early – a lesson old-school operators have drummed into me since day one. Upgrading wastewater treatment so smaller towns and plants stay ahead of tighter water regulations protects both the business and the community around it. Training for line workers, to refresh the basics of chemical handling and spill cleanup, lands high on my list for staying ready in a busy shop.
People using products at home will keep running into SXS, whether they know it or not. Every improvement in safety, clarity, and environmental responsibility gets passed along and builds trust, bit by bit. Watching the supply chain closely, investing in better tech, and asking for transparent labeling keeps this chemical helpful and out of the headlines for the wrong reasons.
Sodium xylene sulfonate turns up a lot in cleaning products, especially shampoos and dish soaps. It helps water and oil mix, making it easier to remove grease or dirt. Plenty of folks at home might recognize it only by its place on the back of a label, but this ingredient gets into our waterways each time you rinse out your sink or take a shower.
Anything we use at home can eventually leave the house and enter the environment. Whether washing floors, doing laundry, or cleaning the car, the soaps send small amounts of sodium xylene sulfonate down the drain. So, the reason for asking about its biodegradability feels straightforward—it’s about figuring out if what we use will break down naturally or stick around where it shouldn’t.
Researchers studying how chemicals behave in the environment have tested sodium xylene sulfonate’s breakdown. They’ve dosed it in different water samples and checked how long microbes take to chew it up. The good news: sodium xylene sulfonate has shown to be “readily biodegradable” under most test conditions. This means that in the presence of bacteria, it gets broken down into simpler, less harmful substances. Various studies have found that, within a few weeks, most of this compound no longer shows up in treated water samples. It doesn’t build up over time because soil and sewage treatment plants contain the right kind of bacteria to take it apart.
Not every environment supports the same level of breakdown. Colder water, poor circulation, or low bacteria counts can slow things down. If sodium xylene sulfonate only partially degrades, fragments might linger and cause problems that no one has studied in depth. This risk grows in rural areas with less advanced treatment or in places with heavy use. So, while the ingredient breaks down quickly at well-managed plants, it won’t always break down at the same speed in wild rivers, lakes, or septic systems.
Regulators and scientists often agree that sodium xylene sulfonate rates as low-to-moderate risk for aquatic life, mostly because it doesn’t stick around once released. The European Chemicals Agency and the U.S. Environmental Protection Agency both have files showing that it breaks down well, and does not bioaccumulate in fish or water plants. This plays a part in earning its approval for widespread commercial use. Some consumer watchdogs have pointed out a lack of large-scale, long-term independent studies, but at the moment, the evidence leans toward good environmental fate after use.
Even biodegradable substances cause problems if used in huge quantities or dumped down storm drains. Being careful about what goes into the environment still matters. There’s always space to push manufacturers and regulators for more robust, real-world testing, especially in diverse geographic regions and treatment conditions. Homeowners with older septic systems might choose lower-impact soaps or seek brands that publish biodegradability proof. Pulling evidence from new fieldwork, not just lab results, would build trust and fill knowledge gaps around this ingredient.
Sodium xylene sulfonate usually breaks down after use, so the risk to rivers and aquatic life stays quite low compared to some stubborn cleaning agents. I’ve worked with wastewater engineers before, and the consensus feels pretty reassuring—given good treatment, this chemical rarely causes headaches. Still, there’s value in keeping up pressure for more research and full transparency from the chemical makers. Our waterways do better when we ask the right questions and demand honest answers.
Every day products—soap, shampoo, laundry detergent—count on chemicals for cleaning power. One often shows up on the list: sodium xylene sulfonate. It doesn't get the same headlines as parabens or phthalates, but it's always quietly there, making things blend together or foam up better. Not many of us read the back of a shampoo bottle and think hard about what each ingredient does. If you have sensitive skin or a history of allergies, you probably want to know whether sodium xylene sulfonate causes trouble.
Sodium xylene sulfonate isn’t the flashiest name, but research on its safety runs deep. The Cosmetic Ingredient Review (CIR) flagged it for review along with similar compounds. They found little evidence of it causing allergic reactions in the general population, although a few cases of mild irritation popped up under intense, undiluted use. Dermatologists haven’t seen an explosion of complaints about this chemical—among thousands of patch tests, only scattered reports of skin irritation came up, with allergy rates lingering at less than 1%. By contrast, preservatives and fragrances draw far more red flags for allergy risks.
Most folks never notice sodium xylene sulfonate. If you have a history of contact dermatitis, eczema, or other sensitivities, any detergent or chemical can leave you red and itchy. My own skin reacts to random soaps—one day nothing, the next dry patches, no rhyme or reason except sometimes an ingredient I haven’t used before or a formula that's too strong. Sodium xylene sulfonate makes it into detergents and cleaners because it helps other ingredients dissolve and spread, not because it causes a tingling sensation or rash.
Still, everyone’s body chemistry runs a little different. Allergic reactions, including contact dermatitis, look like redness, itching, swelling, and sometimes blisters. Usually, doctors look for patterns over time—did it happen right after using that new face wash? Did a patch test cause a rash? The answer points to an irritation, a rare allergy, or a reaction to something else in the product. Sometimes, the combination of chemicals, fragrances, or preservatives does more damage than any one ingredient alone.
Good habits help avoid most problems. If a product stings, burns, or leaves a rash, I toss it, no matter what the label says. Sensitive skin means sticking to short ingredient lists, fragrance-free options, and patch-testing new stuff behind the ear or on the arm. Reading the research helps, but your own skin is the best judge.
For folks who work with cleaning products all day, skin protection goes a long way—gloves, frequent moisturizing, and quick rinse-offs. If you worry about possible allergies to sodium xylene sulfonate, tracking symptoms in a journal helps you figure out which ingredient triggered your reaction, with a doctor or dermatologist guiding the process. Skin patch testing sorts out if sodium xylene sulfonate or something else causes problems.
I’ve seen new brands aim for cleaner labels, skipping out on less familiar chemicals and focusing on what doesn’t upset people’s skin. The European Union keeps tight regulations on product ingredients, and keeps reviewing safety data as more information comes in. Sodium xylene sulfonate will keep showing up in products until safer, proven alternatives come along that clean as well without side effects. The best way forward stays the same: transparency, updated research, and honest conversations between customers, companies, and scientists. Being informed and watching how your body reacts usually keeps you healthier than any fancy slogan on a bottle.
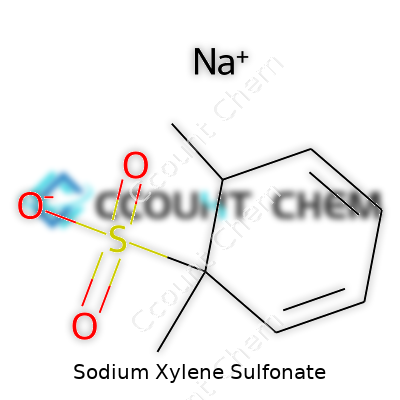
| Names | |
| Preferred IUPAC name | Sodium 3-methylbenzenesulfonate |
| Other names |
SXS Sodium xylol sulfonate Sodium dimethylbenzenesulfonate |
| Pronunciation | /ˌsəʊdiəm zaɪˈliːn sʌlˈfəʊneɪt/ |
| Identifiers | |
| CAS Number | 1300-72-7 |
| Beilstein Reference | 1711319 |
| ChEBI | CHEBI:9120 |
| ChEMBL | CHEMBL38358 |
| ChemSpider | 20219 |
| DrugBank | DB11310 |
| ECHA InfoCard | ECHA InfoCard: 100.010.047 |
| EC Number | X |
| Gmelin Reference | 84237 |
| KEGG | C01601 |
| MeSH | D013007 |
| PubChem CID | 23665739 |
| RTECS number | XW6475000 |
| UNII | Y3V6N0F73Q |
| UN number | UN2309 |
| CompTox Dashboard (EPA) | urn:epa.comptox:dtc0000623 |
| Properties | |
| Chemical formula | C8H11NaO3S |
| Molar mass | C8H9NaO3S: 208.21 g/mol |
| Appearance | Colorless to light yellow transparent liquid |
| Odor | Odorless |
| Density | Density: 1.15 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.9 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 6.2 |
| Basicity (pKb) | 7-8 |
| Magnetic susceptibility (χ) | -23.0e-6 cm³/mol |
| Refractive index (nD) | 1.440 |
| Viscosity | 50 – 250 cP |
| Dipole moment | 8.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 362.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -661.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | Not found |
| Pharmacology | |
| ATC code | V03AE02 |
| Hazards | |
| Main hazards | Causes skin and eye irritation. |
| GHS labelling | GHS07, Warning, H315, H319, H335 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Causes serious eye irritation. |
| Precautionary statements | Wash thoroughly after handling. Wear protective gloves/eye protection/face protection. IF ON SKIN: Wash with plenty of water. If skin irritation occurs: Get medical advice/attention. Take off contaminated clothing and wash it before reuse. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > >100 °C (212 °F) |
| Lethal dose or concentration | LD50 Rat oral 7,200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat) 7200 mg/kg |
| NIOSH | WF1815000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 40% |
| Related compounds | |
| Related compounds |
Xylene Xylenesulfonic acid Sodium toluene sulfonate |