Before most folks today even touched a science textbook, chemists were trying to wrangle useful derivatives from simple organic molecules. As early as the late nineteenth century, scientists saw that turning small, reactive sulfonamide groups into salts like sodium sulphamidate opened doors for both easy handling and new applications. Industrial production took shape in Europe during the growth of synthetic dyes and pharmaceuticals. Sodium sulphamidate, tucked between early antibacterials and large-scale chemical platforms, saw its reach expand in the twentieth century. The need for reliable intermediates in manufacturing meant producers scaled up, improved purification, and worked on standardizing product quality—progress that only happened once businesses actually saw practical value in getting their hands dirty with benchtop chemistry.
Sodium sulphamidate doesn’t just catch the attention of big chemical suppliers; its structure puts the focus on its reactive sulfonamide group made more soluble and manageable by turning it into a sodium salt. People who’ve worked with this compound find it to be a crystalline powder, usually white, that dissolves quickly in water but keeps solid when exposed to normal air. A high-purity sample doesn't throw up red flags on routine testing, which makes it appealing for consistent lab work and industrial uses. This stability lets users count on repeat results, especially if the storage and packaging match technical expectations shaped over decades.
Not all chemical powders act the same, and sodium sulphamidate brings its own profile. The substance forms white to off-white crystals or powder, often spilling from jars with barely any noticeable smell. It doesn’t melt at low temperatures, but with enough heat, it’ll decompose before you see liquid pooling. High solubility in water stands out, allowing easy incorporation into a range of solutions and reaction mixtures. Its molecular formula gives a clean balance of sodium, nitrogen, sulfur, oxygen, and hydrogen—no rare earth surprises or heavy metal contamination. Though stable for storage, handling strong acids or bases will quickly change its structure, so you need to keep an eye on the working environment.
Science doesn’t smile on shortcuts, so technical details matter. Commercial sodium sulphamidate usually hits purity levels above 98%, with sodium, sulfonamide, and moisture content regularly specified. Reliable suppliers show heavy metal levels under 10 ppm, and voluntary details cover residual solvents and organic impurities. Packaging lines stamp bags and bottles with batch numbers, certifications, and safety information. Every legitimate shipment from trusted brands comes with a certificate of analysis matching international and local regulations. In the real world, most buyers I know check for the sodium sulphamidate name but also watch for global synonyms listed on labels, since imports and exports get confusing fast.
Chemical plants pumping out sodium sulphamidate start from chlorosulfonic acid and ammonia, forming sulfonamide, and then bring in sodium carbonate or sodium hydroxide to swap in the sodium ion. Batch and continuous reactors both see use, depending on the production volume. After the main reaction, operators separate the crude solid, wash impurities away with water or ethanol, and finish things up by drying the crystals. Large-scale operations focus on consistent quality, mostly by controlling reaction conditions and monitoring raw material grades. Workers on the line spend years figuring out how much temperature and time tuning leads to fewer side products and less waste.
People familiar with sulfonamides know their value comes from reactive nitrogen and sulfur groups. Sodium sulphamidate makes clean templates for all sorts of N-derivatization and sulfonamide couplings. Strong oxidizers, acids, or alkylating agents won’t leave it untouched. One route, for example, involves attaching complex organic units by replacing the sodium—a well-tested trick in pharmaceutical and material chemistry. In process research, sodium sulphamidate often serves as a bridge to specialty sulfa drugs, and as a group partner in synthesizing dyes, stabilizers, and farm chemicals, sometimes improving selectivity or yield by orders of magnitude compared to older methods.
Depending on where you order or read, sodium sulphamidate carries a handful of names. Sodium aminosulfonate, sodium sulfaminate, and sodium sulfamidate all come up in catalogs and research. Some European suppliers label it differently from counterparts in Asia or North America. Drug formulators check pharmacopoeias for synonyms, careful not to mix up regulatory standards. In manufacturing, workers and buyers stick with CAS numbers to avoid confusion, but the average lab order still reads “sodium sulphamidate” front and center. Older patents and research articles might use legacy names, which sometimes makes tracking applications over decades a headache.
Handling sodium sulphamidate safely means following established chemical hygiene and regulatory limits. Dust can irritate eyes and airways, so proper ventilation and personal protective equipment sit at the core of plant and lab protocols. Training covers safe handling of both the raw substance and process waste streams, since breakdown products can raise risk if inhaled or released without treatment. Workers double-check local environmental release thresholds, and industrial buyers pay close attention to international transport rules for chemical safety. Emergency sheets detail hazard classification, recommended fire response, and steps for accidental spills. Solid experience on the shop floor often prevents issues documented in regulatory guides.
In the hands of skilled chemists, sodium sulphamidate helps launch reactions that build therapeutic agents, industrial dyes, herbicides, and specialty polymers. Its reliable behavior as a nucleophile or base makes it a favorite among process development chemists who push for high yields and a cleaner product stream. Some fields value it for modifying active pharmaceutical ingredients, letting them tune solubility or biological activity in small steps—far easier than starting from scratch. Agricultural firms bring it into pre-plant herbicide synthesis, chasing better results and less off-target effect. The dye and pigment sector taps it for intense color development after sulfonamide modification, especially where regulatory agencies keep tightening purity rules.
Exploration in both academia and industry drives new uses and improved processes for sodium sulphamidate. Analytical research digs into crystal structure, reaction intermediates, and custom synthesis with green chemistry in mind. Modern process labs are looking for cleaner conversion routes, both to boost yield and spare the environment from extra by-products that long-term exposures can bring. As automation pushes into analytical labs, companies use sodium sulphamidate as a consistent standard during scale-up and pilot projects. Biological research circles it for studies on enzyme inhibition or as a scaffold for high-throughput screening, showing the reach of a compound with deep, flexible reactivity.
Public and workplace health researchers keep a close watch on possible hazards related to sodium sulphamidate. Most animal studies point to moderate acute toxicity at high doses, with irritation risk for skin and eyes on direct exposure—common to a lot of sulfonamide derivatives. Chronic toxicity and environmental persistence haven’t set off big alarms, but researchers keep running new in vivo and in vitro tests to catch long-term effects. Environmental chemists follow breakdown in soil and water, particularly in areas downstream from manufacturing plants, hoping to prevent surprises in ecosystems over years. Regulatory agencies in North America and Europe generally classify it as a substance calling for standard industrial care: gloves, goggles, up-to-date training, regular health surveillance.
Anyone looking to where sodium sulphamidate goes next should focus on specialty synthetic chemistry, greener manufacturing, and medicinal innovation. Market forces encourage suppliers to trim waste, lower raw material footprints, and cut emissions tied to sulfonamide salt chemistry. Pharma and diagnostics researchers keep after small, reliable molecules with high substrate specificity, and sodium sulphamidate stays in the running because of its stability and predictable reactions. With interest in sustainable agriculture climbing, new formulations might take root where better crop safety or environmental persistence gives users a practical edge. Down the road, regulatory changes may shift how sodium sulphamidate moves across borders or gets certified for use, but what won’t change is the demand for flexible, robust intermediates that enable breakthrough research and industrial transformation.
Most people never pick up a bottle labeled “Sodium Sulphamidate” at the store, but that doesn’t mean it’s rare. This chemical finds a place in modern life, especially in fields most folks trust but never see up close. Years ago, I learned about it while working with a group that supported water safety. The stuff sits behind the scenes in both medicine and industry.
Doctors sometimes rely on sodium sulphamidate as part of wound-cleansing solutions. Hospitals lean on it to stop bacteria from running wild. It helps manage infection risk for burns, surgical wounds, and some skin conditions. Researchers have spent years testing this chemical, and its record stands out—bacteria find it tough to adapt, making it a good pick where other antibiotics start to fail. Health workers depend on this, especially in crisis zones where infection control can sink or save a plan.
I spoke to a wound care nurse who emphasized the need for options. “Some antibiotics become useless over time,” she said. “Sodium sulphamidate gives us a backup.” This practical approach, supported by clinical studies, proves it earns that spot in the hospital cabinet.
Factories use sodium sulphamidate for more than fighting germs. It works in dyes, chemicals, and water treatment. Textile folks value it for the way it binds to other compounds. Water engineers use it to keep municipal supplies safe, slowing the spread of microbes.
According to a 2022 paper in the Journal of Applied Chemistry, the chemical plays a role not just as an agent in the process but as a stabilizer, cutting risks from impurities. That adds up to better results and fewer costly recalls in finished products.
No chemical comes free of concerns. Safety data sheets ask for gloves and eye shields. Accidental spills on the skin can burn or irritate. Breathing in powder can set off coughing and discomfort. Regulators charge labs and factories with tight standards for handling and disposal. Parents want reassurance—sodium sulphamidate rarely slips into home products, but school labs sometimes keep it around for testing.
Confusion between similar-sounding chemicals has sometimes sparked concern. For example, some people hear “sulphamidate” and worry about allergies linked to sulfa drugs. According to pharmacists, the link mostly matters for people with documented sulfa allergies, a point doctors keep in mind before using it.
Public health wins depend on clear information and solid oversight. Lawmakers could support more frequent training in labs and factories. Transparency in labeling and responsible purchasing helps everyone stay safer. Schools that use chemicals for experiments can teach students about good handling instead of treating it like a secret.
Sodium sulphamidate won’t show up in most shopping carts, but its impact stretches from hospital recovery rooms to water that actually tastes clean. People deserve honest facts—about the benefits, limits, and risks of the chemicals holding today’s world together. By facing the facts and supporting responsible use, communities and professionals can get the most out of what this compound offers.
Anyone who's spent time around chemical stores or labs knows the value of treating every substance with respect. Sodium sulphamidate doesn't spark curiosity because of headline-making incidents, but because even seemingly mild chemicals can set off reactions that leave stains on both shirts and health records. Experience reminds me that small errors can multiply, especially when mixed with a crowded workspace and a distracted mind.
During my college internships, the rule echoed by supervisors always stuck: gloves and goggles come on before any work starts. Sodium sulphamidate’s powder form gets airborne easily, drifting into eyes or onto skin. Direct contact can lead to irritation, and inhaling dust never ends well. Those cheap nitrile gloves and well-fitting goggles do more than just satisfy protocol. Once, I saw a classmate skip eye protection for a “quick transfer” and spend the afternoon at the campus clinic. Lab coats keep sleeves clean, but they also add a barrier no one thinks much about until after a near-miss.
Not every workspace has shiny fume hoods, but air movement matters. Open containers, careless scooping, or a running fan can send particles everywhere. I learned quickly to mix chemicals under the hood or at least in a spot with good cross-ventilation. Even a mask, the kind everyone knows from dusting or painting, blocks most particles. Skipping this step means carrying home more than just your lunch.
Shelves fill up easily, and in the rush to finish up, it’s tempting to reach for an unmarked bag or bottle. “It’s just leftover,” I once heard, but that “just” can cause confusion. Mishaps often trace back to lost labels or faded handwriting. Take thirty seconds for a fresh label. List the date, concentration, and your name. Best habits come from the urge to protect not just yourself but the next tired intern arriving before dawn.
The sticky residues of sodium sulphamidate can linger, hiding in corners of balances, under trays, or around lids. I learned to wipe down benches and scales as I move, not just at the end of the day. Spillage happens with powders, and a damp cloth picks up more than dry paper. Hazards become invisible when routine cleaning gets skipped.
No one expects spills, but they happen. It helps to know exactly where the eyewash, shower, and first aid kit stand. Walking the route once embeds it in memory. Supervisors often remind staff during safety drills, but real emergencies don’t wait for reminders. If a spill hits your hands or face, quick rinsing trumps hesitation.
Waste bins fill up fast, and throwing everything together invites trouble. Sodium sulphamidate joins other chemical wastes that health and safety offices want separated. Dedicated bins, sturdy liners, and clear signage help, but the real rule is: never dump or wash chemical waste down the drain. Ask about local disposal protocols and follow them. Fines hurt, but environmental damage lasts longer.
Training and procedures help, but habits and mindfulness matter more. Sharing stories of near-misses with new team members keeps safety more than a checklist. Sodium sulphamidate isn’t some villain, but good safety respects chemicals—all chemicals. Trusting instincts, looking out for your team, and double-checking every step brings everyone home healthy.
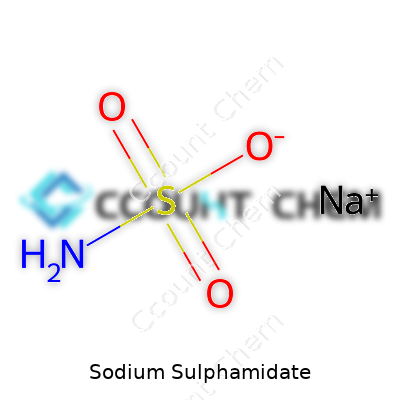
People working in science or industries using chemicals have probably come across sodium sulphamidate. This compound runs with the chemical formula NaSO2NH2. Sodium comes together with the sulphamidate group, creating a solid that appears as a white, crystalline powder. That formula isn’t just a string of elements. It stands as a promise: a blueprint for the way atoms organize and interact with one another in the real world.
I’ve come across sodium sulphamidate mainly in water treatment and pharmaceuticals. It’s known for cleaning up water by removing unwanted metals and stopping bacteria. Some reports point to uses in dye production or medicine recipes. Knowing its formula isn't just about answering a test question. It's about practical safety, handling, and knowing exactly what you’re working with. Without this basic information, mistakes in storage or mixing can turn serious quickly.
Understanding the formula turns into a safety check. Sodium can react strongly with water; add it carelessly, and you risk a reaction you didn't plan for. The -SO2NH2 group makes the compound more stable, but it can still release ammonia or sulfur dioxide if it breaks down. Breathing in those gases isn’t just unpleasant – long-term, it can cause coughs, headaches, and worse. Chemical accidents often trace back to someone not understanding what was in that bag or bottle. Simple awareness about NaSO2NH2’s formula cuts those risks.
Good water is no small matter; millions rely on chemical treatments to make it safe. Sodium sulphamidate helps get rid of dangerous metals, especially in rural and industrial regions. Pharmaceuticals lean on similar substances to keep products pure and safe for folks who need medicine. If the chemical composition isn’t checked at every step, entire batches can be contaminated or lose effectiveness. I’ve seen this with other compounds where one wrong calculation led to wasted time and resources.
Science isn’t only about memorizing formulas. But skipping over basics like NaSO2NH2 leaves new workers guessing. At a water facility or drug plant, those errors don’t just cost money—they can cost public trust, even health. Universities and training programs do best when they ground theory in lab experience. Simple exercises—measuring out grams, seeing real reactions—build confidence and judgment better than any memorized chart. Mentors I’ve worked with often explained not just the “what,” but the “why” behind every name and label.
Everyone who works with things like sodium sulphamidate needs clear labels, honest reporting, and regular safety drills. Labels should list chemical formulas, main hazards, and emergency advice. Companies can do more by sharing incident stories—anonymized, but real—so nobody repeats old mistakes. Household products rarely include things like sodium sulphamidate, but in industrial settings, routine inspections and double-checks keep problems from growing. At the end of the day, understanding a formula like NaSO2NH2 takes shape not just in the classroom, but in every careful scoop, pour, and mix that keeps the world running.
Even simple chemicals like sodium sulphamidate deserve respect in storage. Over the years, I’ve seen what happens when folks take shortcuts. You end up with damaged stock, wasted money, or worse—a hazardous incident. Your best bet always begins with understanding the chemical’s quirks. Sodium sulphamidate absorbs moisture fast, and any trace of water starts its slow breakdown. I never leave such chemicals in a warm, damp utility closet or near the steamy kettle in the break room. Cool, dry shelves with just enough circulation do far more for peace of mind.
Most issues with sodium sulphamidate begin at the seal. Bags left ajar or reused plastic tubs don’t cut it. Even after one brief opening, always make sure lids clamp tight and the seal shows no cracks. I recommend containers with a screw cap or lever lock, made from sturdy, chemical-resistant plastic or glass. You want to keep humidity and dust away. Somebody once used an old food jar to store a leftover batch. That jar ended up leaching oil into the chemical—the batch went into the hazardous waste bin faster than you can say “surplus.”
Sodium sulphamidate doesn’t react well to light or heat. Direct sunlight from a window or the heat of nearby machinery can speed up degradation. In one cramped stockroom I saw, someone left a carton too close to a radiator. Within a week, the powder had clumped together and the odor shifted. If the label says “store below 25°C,” keep it there. Dark cabinets and insulated cupboards serve best. Acids can trigger nasty reactions. Store the sodium sulphamidate on a different shelf entirely, not only across the aisle from cleaning products and acidic glues. One careless mix-up can mean strong-smelling gas and a halt to everyone’s workday.
Proper labeling goes a long way. Write the date received and opened. Chemicals spoil quietly. If expiry sneaks up and you haven’t checked, you may risk product failure in your next process. Rotating stock—using the oldest first—costs nothing and avoids surprises. I’ve seen seasoned techs treat the label as an afterthought, leading to mistaken identity and near-misses. Nobody wants to stand down a line to check a powder under the microscope—or worse, after a risky reaction. Keep records simple but clear, and clue in your team on expiry tracking.
Spills happen, even for the careful. Planning ahead means stocking gloves, goggles, and a broom just for chemical clean-ups. Vacuuming up fine powders just spreads the mess. Shoveling into labeled waste pails keeps disposal legal and safe. Local regulations vary, but most waste services ask for sodium sulphamidate to stay sealed until pickup. Don’t pour leftovers down the drain, no matter how much you want a quick fix.
I’d rather lose an hour to careful storage than a day dealing with a spill or a health scare. Solid habits—dry air, tight seals, smart labeling—keep both people and products in top shape. Training every team member, not just the supervisor, keeps mistakes rare and effects minor. If unsure, consult manufacturers’ safety sheets or ring a specialist. Safe storage isn’t just ticking rules; it builds trust and saves on headaches. Even in small labs, this approach pays off every time.
Sodium sulphamidate rarely attracts headlines, but it finds use in all kinds of places—textile production, pharmaceuticals, and even water treatment. Ever notice how many chemicals in our daily lives fly under the radar? The thing about sodium sulphamidate: people who work with it every day face more risks than the general public wandering by a manufacturing plant.
Breathing it in or letting it touch the skin, that’s where trouble can start. If you check out industrial hygiene resources, you’ll find plenty of stories about chemical burns, breathing problems, or rashes that show up after contact. Workers get the worst of it. No one wants to end a shift with a persistent cough or a stained, through-the-glove injury. That's why industry organizations push for heavy use of masks, gloves, and proper ventilation where this stuff is found.
From a consumer’s angle, most people don’t see sodium sulphamidate labeled on household products. It tends to show up far upstream in manufacturing, meaning the direct exposure in everyday life lands closer to zero for most folks. If you live near a facility using or producing it, local regulations matter a lot. Testing the air and water isn’t busywork; it’s what lets the neighborhood know their health isn’t trading places for industrial profits.
Science says sodium sulphamidate doesn't build up inside the body like heavy metals. Still, toxicity reports agree—even one bad spill in a closed workspace mimics the harm you’d see from more widely known irritants. The Environmental Protection Agency and the European Chemicals Agency flag it for its potential to cause serious eye and skin damage, especially in concentrated forms.
So far, long-term cancer risks from this chemical don’t draw much concern among toxicologists, but repeated short-term exposures can pile up and leave scars. I remember a local case where improper storage led to a bucket breaking open, filling the air with a powder that caused real health problems for three workers. It left the company scrambling, and neighbors left wondering if they needed to worry about their own water.
Dumping or spilling sodium sulphamidate into rivers or soil? That’s where bigger damage looms. Aquatic life reacts quickly to water changes; small fish and invertebrates tend to die off before any government steps in. If the chemical travels with run-off, suddenly more than one neighborhood feels the hit. Using earth-based remediation—think activated carbon filters or bioremediation beds—can catch some of the contaminants before they spread.
Nobody likes reading about rivers full of dead fish or farmers who can’t use their water for a season. Regions with modern chemical management keep a pretty close eye on effluent going into surface water, but not every factory worldwide can say the same.
Safer workplaces start with proper training. Teaching workers about real-life consequences—blisters, trouble breathing, groundwater complaints—sticks more than warnings about chemical names and exposure limits. Automation helps limit exposure, and investment in closed systems over open-air vats reduces the accidents people never forget.
For the environment, local laws need teeth, not just paper promises. Reporting spills, quick cleanup protocols, and third-party inspection make a difference, and communities near industrial sites should know what chemicals move through their area. Information shouldn’t live only in company records. Every family, school, and small business deserves to know what’s in their air and water, and what to do if danger shows up.
| Names | |
| Preferred IUPAC name | Sodium sulfamate |
| Other names |
Sulfanilic acid sodium salt Sodium p-aminobenzenesulfonate Sodium sulfanilate |
| Pronunciation | /ˌsəʊdiəm sʌlˈfeɪmɪdeɪt/ |
| Identifiers | |
| CAS Number | 138-88-5 |
| 3D model (JSmol) | `data="C1(=NC(=O)NS(=O)(=O)O)C=O"` |
| Beilstein Reference | 1438463 |
| ChEBI | CHEBI:9124 |
| ChEMBL | CHEMBL2105972 |
| ChemSpider | 44930 |
| DrugBank | DB09294 |
| ECHA InfoCard | 03e2e0de-1f5d-4634-8815-1da10eb3196b |
| EC Number | 238-007-6 |
| Gmelin Reference | 153948 |
| KEGG | C14351 |
| MeSH | D013526 |
| PubChem CID | 25081212 |
| RTECS number | WO8400000 |
| UNII | CN7D40I6F7 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | NaSO2NH2 |
| Molar mass | 124.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.49 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.1 |
| Basicity (pKb) | 10.06 |
| Magnetic susceptibility (χ) | -47.0e-6 cm³/mol |
| Refractive index (nD) | 1.445 |
| Dipole moment | 3.35 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 107 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -489.77 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1156 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | S01AX04 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory irritation; harmful if swallowed. |
| GHS labelling | GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 oral rat 3500 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1000 mg/kg (oral, rat) |
| NIOSH | WF4010000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.5 mg/m³ |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
Sulfanilamide Sulfanilic acid Sodium sulfanilate Sulfanilamide sodium salt |