Sodium sulfamate caught the scientific world’s eye back in the early twentieth century, right when industries were starting to tinker with nitrogen-based compounds. Chemists learned to harness this salt for its unique properties after basic amines became popular in various cleaning and chemical processes. Back in those days, laboratory workers mixed sulfur trioxide with ammonia, chasing new ways to clean equipment, soften fibers, and treat water. Stories of large industrial plants trying to squeeze every ounce of efficiency from raw materials usually circle around utility chemicals like sodium sulfamate; the stuff helped drive chemical manufacturing efficiency, and it's still in use for much the same reason. This material filled niches that other products missed, supporting everything from textile finishing to sugar refining as new uses were found.
Most people run across sodium sulfamate in technical chemistry labs, industrial plants, or certain specialty cleaning products. It looks like a white, odorless crystalline powder that dissolves readily in water and doesn’t leave behind much residue. The compound’s stability and strong sulfamic acid base make it handy for tailored purification steps, pH adjustments, and large-scale syntheses. Among industrial chemists, sodium sulfamate lines the shelves as both a cleaning solution ingredient and a versatile reactant. While folks outside these circles rarely notice, the presence of this compound in day-to-day products reveals how deeply woven these salts have become in solving everyday challenges, from keeping surfaces scale-free to ensuring metal doesn’t corrode in high-stress environments.
A closer look shows sodium sulfamate as a crystalline solid, sitting at around 217-220°C before it breaks down. Water welcomes it in; you’ll get a clear solution, which makes handling straightforward for large-scale users. Chemically, it brings both acidity and strong nucleophilic traits to the table — handy for hydrolysis and neutralization reactions. The sodium ion delivers its own utility, ensuring the salt won’t gum up or cake under normal storage conditions. The lack of a strong odor helps too, especially for operators bothered by pungent industrial chemicals. More importantly, its physical traits mean workers can measure, mix, and clean up after using sodium sulfamate without headaches, since the residue won’t foul up pipes or tanks.
Chemical manufacturers set standards for sodium sulfamate, looking at purity levels, water content, and free acidity. Purity above 99% fits the high demands of industrial production. Specs highlight the absence of heavy metals and offer low chloride numbers. Packaging often features sturdy, moisture-proof bags or drums labeled with chemical identifiers, hazard pictograms, and handling instructions. Storage tips pop up all over datasheets, suggesting cool, dry places away from acids and oxidizers. Labels focus on keeping operators aware of eye and skin irritation risks, while clear instructions cut down on mistakes during dosing or transfer.
The standard recipe for sodium sulfamate starts with sulfamic acid and a sodium base, often sodium hydroxide or carbonate. Sulfamic acid dissolves in water, followed by slow, careful neutralization with the sodium source. The mixture is stirred, monitored for pH, and usually warmed to ensure complete reaction. Any impurities come to the surface and get filtered out. The resulting solution concentrates, then crystallizes by evaporation or cooling. Factories with good control practices wash and dry the finished solid, then pack it up while mindful of keeping moisture at bay. The preparation’s straightforward steps dovetail with scale-up—large reactors make it possible for industrial batch size to stretch from a few liters all the way up to tons in commercial-scale plants.
Sodium sulfamate works like a blank canvas for clever chemists, reacting with plenty of acids, bases, and oxidizers. Its ability to donate a sulfamoyl group opens up the path for synthesizing sulfonamides and other nitrogen-based specialty chemicals. In presence of chlorine or bromine, sodium sulfamate can form N-halogenated derivatives, which serve as disinfectants and bleaching agents. Reacting it with organic halides sometimes leads to medicinal intermediates. Its gentle reaction profile, compared to more volatile chemicals, gives a safety net—less risk of runaway heat or toxic byproducts. Chemical engineers rely on its stability to prevent unexpected breakdowns during long, multi-step syntheses, which stretches its value far beyond a mere cleaning aid.
Industry buyers and researchers may encounter sodium sulfamate under a handful of names: sodium amidosulfonate, sodium sulfamic acid, or its systematic listing as a sodium salt of sulfamic acid. Synonyms crop up in supplier catalogs, but all point to the same essential ingredient. Trademarked products might blend it with other salts or specialty additives, sometimes branded for use in descaling, paper bleaching, or textile softening applications. No matter the label, the chemical backbone remains the same, helping buyers make informed choices about composition and purity without chasing after obscured technical data.
Plant operators and researchers treat sodium sulfamate with the typical respect for mid-strength industrial chemicals. The material brings irritation risk if it contacts eyes or broken skin, though it doesn’t carry the inhalation dangers seen in some stronger acids. Standard safety data sheets highlight gloves, goggles, and good ventilation in work areas. In storage, keeping moisture out prevents clumping and reduces the risk of accidental hydrolysis over time. Disposal recommendations steer toward dilution and neutralization, with stormwater protection guidelines in place to keep runoff away from drains and waterways. US and EU regulatory agencies classify it away from the worst hazards, though enterprises keep safety culture strong to avoid slips.
Its track record in water treatment plants rivals its use in industrial cleaning solutions; the compound breaks down scale and mineral deposits, letting pipes and heat exchangers run longer with less downtime. Textile finishing outfits use sodium sulfamate to soften fibers and prep materials for dyeing or further chemical treatments. In printed circuit board manufacturing, the compound sometimes acts as an etching agent. Sugar processing, leather tanning, and paper bleaching also turn to this salt, leveraging its moderate reactivity and clean breakdown. The versatility extends even to pharmaceutical intermediates, where it paves the way for cleaner, more selective syntheses of sulfonamide drugs, all without burdening the process with heavy metals or troublesome byproducts.
Lab teams keep investigating sodium sulfamate for ever more selective organic transformations. The compound has grown into a springboard for exploring new functional groups and reaction mechanisms in green chemistry initiatives. Improvements keep rolling in, especially with scientists testing milder conditions for synthesis and better integration with renewable feedstocks. Studies dig into its ability to stabilize halogen intermediates and its role as a soft oxidant, lowering process temperatures and, by extension, energy costs. The learning from decades of accumulated R&D pushes its boundaries—sometimes revealing new catalytic functions or ways to limit environmental footprints in otherwise energy-hungry sectors.
Toxicological reviews on sodium sulfamate paint a low-hazard profile in controlled scenarios, though direct ingestion or heavy skin exposure can lead to irritation or digestive upset. Animal studies point toward medium-level acute toxicity, but lower risk than many inorganic salts. Environmental surveys monitored breakdown products in water treatment and found minimal accumulation risks at standard discharge levels. Testing so far finds the compound breaks down before entering food chains, though environmental scientists watch for chronic exposure in sensitive ecosystems. Medical literature reflects its benign nature, confirming the safety record of properly labeled formulations handled by trained personnel according to posted guidelines.
Renewable industries may push sodium sulfamate into fresh territory, with researchers focusing on reactions that need selective amination or mild sulfonation steps using less energy. Scale-up trials are already underway in large-scale green chemistry programs, aiming to cut down carbon footprints. Digital process control and better logistics reduce both waste and cost, making new applications feasible in advanced composites, bioplastic synthesis, and eco-friendly dyes. Industry analysts see potential expansion in water treatment for arid regions, where scale control brings both cost savings and sustainability. The continued shift toward circular economies and tighter safety regulations should strengthen its market demand, folding sustainability goals into the same chemistry that originally won this material its place in core industries.
Walking past a factory or flipping through cleaning product ingredients, few people stop to consider what’s actually behind the names listed in tiny print. Sodium sulfamate hardly stands out on a label, but it does work quietly behind the scenes across key industries. I’ve spent years around industrial sites, and I’ve watched as this compound moved from a specialty chemical to something hard to replace in daily operations.
Factories get messy. Metal machinery collects scale, rust, and grime. Sodium sulfamate gets mixed with water to remove stubborn mineral build-up from pipes and boilers without eating away at the metal underneath. Engineers and maintenance crews rely on it to keep equipment running smooth, cutting costs and reducing downtime. Steam-driven power plants and municipal water systems depend on this kind of maintenance. Left unchecked, hard deposits force heavy repairs and waste both time and money.
Weeds cause headaches on both highways and farmland. I’ve seen road crews spend days spraying chemical solutions to control overgrown brush along highways and rail lines. Sodium sulfamate stops stubborn weeds where mowing can’t reach or machines risk damaging other infrastructure. In forestry and agriculture, it can help manage invasive growth. Using harsh herbicides carries environmental risks; sodium sulfamate, used responsibly, minimizes persistent residues, helping protect soil quality and nearby water.
It’s easy to forget many household goods come from complex chemical processes. Sodium sulfamate acts as a stabilizer in the creation of colorants and dyes, especially for paper and textiles. Think about how many shirts never lose color after months of washing or how office paper stays bright white. These results stem from careful chemical formulation, not just better raw materials. Even in the world of cleaners, certain formulations use it for safer, more effective products around the house.
Working around chemical plants, I’ve always seen strict rules governing how sodium sulfamate gets handled and stored. If mishandled, it can irritate skin or eyes, and no one wants those accidents on the job. Good ventilation, gloves, and eye protection are basic steps that keep workers safe. Organizations like OSHA and EPA set exposure limits and waste disposal guidelines. Companies investing in training and proper labeling end up with fewer workplace incidents and lower insurance claims. Careless use upstream leads to headaches for everyone downstream.
People often ask if simpler, greener alternatives could replace a chemical like sodium sulfamate. Some new products arrive on the market promising better safety or less impact, but very few match its track record in performance and price. Researchers keep testing bio-based options and innovative blends, searching for ways to get the same results with less risk. Strong oversight, transparent ingredient labeling, and open research help maintain trust while letting industries stay productive. Nothing beats a proven solution, but history shows the most valuable tools always adapt with new knowledge.
Sodium sulfamate shows up most often behind the scenes. It acts as a cleaning agent, a descaler for pipes, and sometimes finds its way into the textile industry. It doesn't make headlines like some harsh chemicals, but that doesn’t mean it’s just another powder you can toss around without care.
Think about baking soda in your kitchen. Easy, right? Now, picture something with a label that sports hazard symbols. Sodium sulfamate often gets that warning. I've seen coworkers assume all white powders act the same. That mistake can leave you with irritated skin or eyes, maybe even shortness of breath if you inhale the dust. The product data sheets — the ones no one likes to read — spell out risks that are worth attention.
No one likes itchy hands or watery eyes at work. Sodium sulfamate can cause those things, usually because people decide gloves or goggles slow them down. Getting even a small amount in your eyes means a direct trip to the eyewash station. Swallowing it, even a tiny bit, means you call in a professional. That information isn’t meant to scare, but to call up a little common sense. If a compound needs a Material Safety Data Sheet (MSDS), give it respect. The science matches up with the personal experience.
Groups like the U.S. Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH) keep their focus on these chemicals. Their job isn’t to invent rules for fun — it’s the result of incident reports, real burns, and allergic responses. They recommend good ventilation, face masks if you’re making dust, gloves, and glasses. Manufacturers echo those protocol because, frankly, nobody wants a lawsuit when someone ends up in the emergency room.
The American Conference of Governmental Industrial Hygienists (ACGIH) also reviews workplace use. Sodium sulfamate doesn’t appear on their most hazardous lists, but they note it causes irritation and sometimes allergies with repeated exposure. Crowded supply closets and bag spills in maintenance rooms can really raise the odds of someone getting exposed.
I’ve cleaned up enough spills to know: dry powder spreads fast, even outdoors. Open windows or use extractor fans to keep dust to a minimum. If the powder lands on your skin, wash right away — don’t wait for a tingling or itching to start. Proper storage keeps it out of reach of visitors or untrained hands. If you’re the supervisor, set up training sessions. Walk through safety gear. Remind teams about clean-up rules. Most incidents begin with a shortcut or forgetting the basics.
I’ve learned to keep personal protective equipment simple and easy to access. Wall-mounted glove racks, goggles hanging by the door, simple signage pointing to first aid supplies — small steps make people more likely to suit up or rinse off after a spill. Spill kits and eye washes come cheap when compared to lost sick days or emergency calls for an exposed worker.
Sodium sulfamate doesn’t bring the same risks as strong acids or inhaled solvents, but brushing off its hazards doesn’t pay off. Personal experience lines up with what the official guidance suggests: know what you’re handling, wear the right protection, and keep instructions in plain sight. If the workplace sticks to those basics, handling sodium sulfamate turns into just another routine that keeps everyone healthy and the job running smooth. Trusting both experience and hard data helps steer clear of unnecessary accidents.
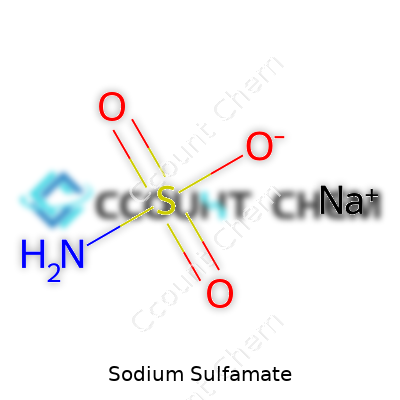
Growing up surrounded by stories about lab mishaps, sodium sulfamate never made its way onto my list of chemicals to fear. It’s a white crystalline powder, easy to dissolve, and far more forgiving than the more notorious substances you might encounter in a high school chemistry demo. If you’re curious, the chemical formula goes like this: NaSO3NH2. While that string of letters and numbers looks like a code to crack, the meaning opens up when you step into the real world of chemistry—one based on needs ranging from metalworking to cleaning up plant roots for weed control.
Sodium sulfamate lands a spot in many businesses, especially where harsh cleaning or weed control plays a role. Take plumbing or metal finishing—sodium sulfamate helps remove stubborn calcium deposits. In my first house, laboring over a clogged pipe would have dragged on forever if the plumber hadn’t scooped out some sodium sulfamate. The formula, with sodium (Na), a sulfamoyl group (SO3NH2), and that single sulfur atom, works to disrupt mineral buildup quickly.
In gardens, professionals value sodium sulfamate’s weed-killing strength. Instead of spraying chemicals all over, they apply it directly to cut stumps, where it targets tough plants right at their roots. It cuts down on unnecessary harm to other plants and reduces lingering toxins in the soil. In a world that tends to throw strong herbicides around eagerly, sodium sulfamate’s targeted approach feels like a small win for anyone aiming to keep soil healthy.
There’s a twist to every chemical’s story: safety comes only with respect and understanding, not just a set of safety data sheets. Misused, sodium sulfamate can irritate the skin, eyes, and airways. Inhaling powder or getting it on hands can result in discomfort or worse, so gloves and a mask belong with the tool kit. Most accidental exposures that cross the bench in local clinics involve lack of preparation—no goggles, no gloves—or folks convinced they “probably won’t need” precautions until it’s too late.
Regulations around sodium sulfamate aim to keep the worst-case scenarios rare. Public records from the Environmental Protection Agency show regular reviews for safe application, overseen by experts who value both efficiency and caution. Experienced users stay sharp by tracking every update, reflecting real-life stories in their safety habits. At work and home, labeling containers and storing sodium sulfamate away from pets, children, and food makes mishaps far less likely.
As technology evolves and sustainability rises on priority lists, chemists and manufacturers face pressure to find safer, cleaner alternatives or adaptations. Sodium sulfamate continues to serve genuine needs, but it becomes even more valuable as part of a bigger strategy: use only what’s needed, combine with training, and never take safety shortcuts. Those steps make its chemical formula more than letters and numbers on a bottle—they define responsible action in industries and homes alike.
Anyone who has spent hours in a lab mixing chemicals understands that some compounds prefer to stay out of the limelight—and by that, I mean light, heat, and moisture. Sodium sulfamate falls into the group where careless habits quickly invite trouble. Used for a range of applications, from herbicide production to cleaning agents, this salt won't start shouting at you, but it needs care to avoid headaches or hazards.
Sodium sulfamate behaves best in dry, cool spaces with good ventilation. Heat turns it stubborn, and moisture inspires clumping or worse, unwanted reactions. A temperature range below 30°C keeps problems at bay, and using desiccants helps shield the chemical from ambient moisture. Forget leaving it near steam lines or hot machinery. Every lab tech I know who ignored this trade-off paid for it in wasted chemicals and cleanup time.
Standard advice says to lean on containers made from polyethylene, polypropylene, or glass. I found out early that metal cans only ask for corrosion. Once, a supplier sent a delivery packed inside a rusty tin. The result was not pretty.
Try to keep the original container if possible. Back in my years at a municipal lab, repackaging drove up contamination risks and spilled chemicals. If a transfer absolutely can’t be avoided, use a clearly labeled, tamper-proof bottle with a tight-fitting seal. It doesn’t seem important until a mix-up costs you hours retracing a batch.
It’s amazing how many headaches proper labeling avoids. One quick glance at a clear label gives you composition, hazard class, and emergency contacts. In workplaces following OSHA or GHS rules, unmarked bottles turn into compliance violations—and can send someone to the ER in a worst-case scenario.
Personal habit: I keep waterproof labels and a black marker handy. Once, a minor leak erased a handwritten label and left everyone guessing if the white powder was sodium sulfamate or ammonium nitrate. That near miss taught me waterproofing isn’t only for outdoor gear.
Sodium sulfamate prefers solitude. Store it away from oxidizers and acids—mixing up neighbors can lead to fires, toxic fumes, and ruined storage rooms. A shelf with other stable, non-reactive salts works well, but never set it near an acid cabinet. Chemical safety rules call this segregation, but most of us call it avoiding disaster. One glance around any veteran’s workspace and you’ll see tape markings or color codes to keep things simple.
If spill control kits or absorbent pads aren’t nearby, you’re playing with fire. Even seasoned handlers can drop a bag, and having the right supplies within arm’s reach shrinks cleanup time and exposure risk. In my lab, a spill kit by every chemical shelf prevented panic more than once. Add gloves, goggles, and a dust mask; you’ll never regret a little extra protection.
Regular checks on seals, labels, and storage space cut down on forgotten stock and decay. Take a few minutes each week to run through the shelving. I’ve seen too many surprises from overlooked jars at the back of the storeroom—clumpy, yellowed, or corroded—mostly from skipping this habit.
Safe storage builds confidence in your workspace. Most accidents stem from shortcuts or overconfidence, not from unforeseen chemical tantrums. With a little planning and basic organization, handling sodium sulfamate turns routine—not risky—and the whole team gets to go home healthy.
If you spill a handful of sodium sulfamate onto a clean table, you notice a white, granular powder, almost like finer table salt. Nobody will mistake it for sugar—there’s a faint chemical smell if you hold it close, nothing harsh, but distinct enough to make you keep it away from food. Sitting on your palm, the powder feels dry. Unlike some powders that clump or stick, sodium sulfamate falls away easily, showing it doesn’t pull moisture from the air rapidly. This low hygroscopicity helps with storage and makes it easy to weigh and measure without worrying about clumps or lumps messing up your calculations in a lab or factory setting.
This salt dissolves in water without fanfare. Stir a spoonful into a glass, and it breaks up with no fuss—even if the water temperature isn’t hot. Solutions with sodium sulfamate turn out clear, with little to no residue. The solubility sits around 210 grams per liter at room temperature, according to published chemical data. In plain language, this means you can make a pretty concentrated liquid without needing to heat it up or wait for ages. For anyone mixing chemicals, or cleaning metals where it’s used as an agent to remove unwanted minerals, this property proves valuable for fast, even dispersal.
Leave sodium sulfamate on a shelf in a dry place, and it keeps its integrity for months. It won’t break down in normal conditions and resists reacting with oxygen or water in the air. Heat it to extremes, though—over 205°C—and it starts to decompose. The key word here is 'decompose', not 'melt'. Unlike table salt, you can’t heat it for cooking experiments or melting; it breaks down and gives off sulfur dioxide and nitrogen compounds. That’s the reason most of us will never see it in liquid form—the solid’s stability gives it a long shelf life if handled correctly.
Much of what makes sodium sulfamate useful ties into these straightforward physical traits. Its neutral pH in water means it doesn’t corrode most metals, lending itself to cleaning and descaling. I’ve seen it recommended for clearing tough scale from industrial boilers without eating away at the pipes. It won’t ignite easily, either, lowering risks in storage and handling. These specifics are critical in a real-world context, where uncontrolled reactions or unpredictable material can drive up costs and risks. Getting a white, odorless, stable powder that’s soluble when needed—it’s a combination sought by chemists and engineers.
Though not especially toxic, sodium sulfamate still demands careful handling. Every chemist I know keeps gloves on while dealing with any chemical powder, and this one is no different. Dusty air or eyes are never fun, and the irritation risk here is real. Keep it dry, keep it in a closed jar, and treat it with the respect you give all fine powders—not just for quality assurance but for worker safety. I’ve always argued that being able to store it for the long haul without it breaking down brings both cost savings and peace of mind for anyone maintaining a supply.
Jobs relying on sodium sulfamate—from plumbing to papermaking—depend on its steady physical behavior. Whether it’s being mixed up for industrial treatment or cleaning, its reliable solubility and shelf life help avoid wasted product. Knowing how it behaves lets users predict outcomes and spot potential problems, which is how you catch mistakes before they cause costly delays. That’s the lesson I’ve taken from my own time watching professionals in different fields lean on this material. Good data on physical properties isn’t just academic—it’s about getting dependable results in daily work.
| Names | |
| Preferred IUPAC name | Sodium sulfamate |
| Other names |
Sulfamic acid sodium salt Sodium sulfamate Sodium amidosulfate Sodium amidosulfonate |
| Pronunciation | /ˈsəʊ.di.əm ˈsʌl.fə.meɪt/ |
| Identifiers | |
| CAS Number | 13845-18-6 |
| Beilstein Reference | 1407932 |
| ChEBI | CHEBI:74841 |
| ChEMBL | CHEMBL1200374 |
| ChemSpider | 28156 |
| DrugBank | DB11169 |
| ECHA InfoCard | 100.016.649 |
| EC Number | 214-603-3 |
| Gmelin Reference | 9030 |
| KEGG | C14326 |
| MeSH | D013008 |
| PubChem CID | 23666376 |
| RTECS number | WA2630000 |
| UNII | 84IAY4CT3V |
| UN number | UN2967 |
| Properties | |
| Chemical formula | NaSO₃NH₂ |
| Molar mass | 97.09 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.48 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | Acidity (pKa): 1.0 |
| Basicity (pKb) | pKb ≈ 7.84 |
| Magnetic susceptibility (χ) | -36.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.485 |
| Dipole moment | 3.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 87.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -538.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1015 kJ/mol |
| Pharmacology | |
| ATC code | V03AB24 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P261, P264, P271, P304+P340, P305+P351+P338, P312, P337+P313, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Autoignition temperature | 400°C |
| Lethal dose or concentration | LD50 oral rat 2480 mg/kg |
| LD50 (median dose) | 1312 mg/kg (rat, oral) |
| NIOSH | RN 5329 |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 30 mg/L |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Sulfamic acid Potassium sulfamate Ammonium sulfamate |