Sodium octane-1-sulphonate monohydrate arrived on the chemical scene around the mid-20th century, during a period marked by rapid advancements in surfactant chemistry and the growing need for reliable chromatographic agents. Analytical laboratories in the fields of pharmaceutical and environmental research looked for solutions that improved separation techniques. Chemists refined the structure of simple alkyl sulphonates, aiming for options that balanced hydrophobic and ionic properties. As reversed-phase high-performance liquid chromatography (HPLC) gained popularity through the 1970s, the need for ion-pairing reagents like sodium octane-1-sulphonate monohydrate became clearer. This compound answered calls for greater retention and selectivity, especially in separating basic drugs and peptides. The journey from early uses as an emulsifier to a staple in advanced analytical methods mirrors the progress of both chemical engineering and quality control across many industries.
Sodium octane-1-sulphonate monohydrate serves primarily as an ion-pair reagent and surfactant. Its key appeal lies in its ability to modify surface tension in aqueous systems, and its role in chromatography brings a level of reliability in separating complex mixtures. White to off-white crystalline appearance, decent solubility in water, and stable shelf life combine practicality with safety. Packing typically uses moisture-barrier containers, given its mild hygroscopic nature. From personal experience working with HPLC, a fresh, correctly stored batch ensures confidence in reproducibility of results, affecting everything from regulatory compliance to patient safety.
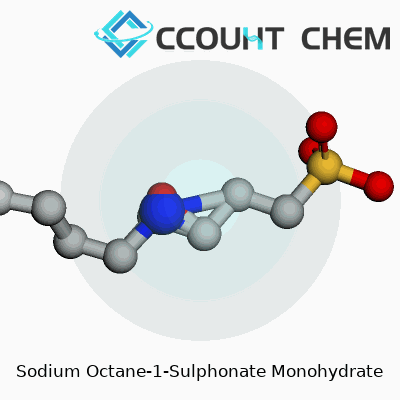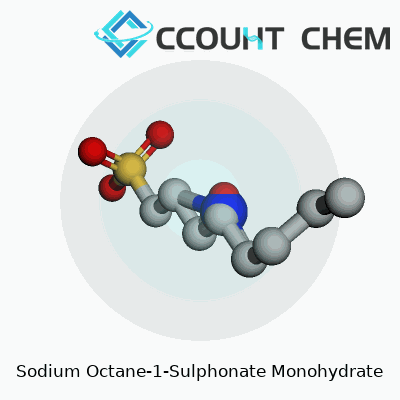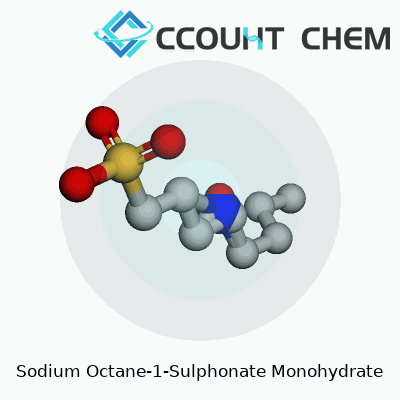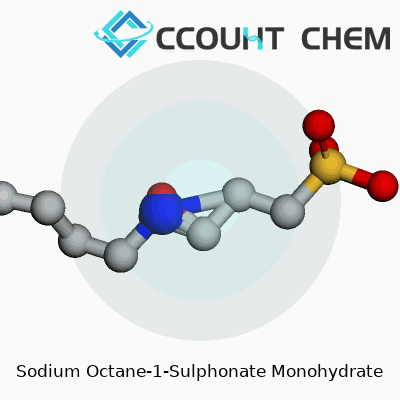
The chemical formula runs C8H17NaO3S·H2O, and the molar mass hovers around 240 grams per mole. At room temperature, sodium octane-1-sulphonate monohydrate appears as a crystalline powder, somewhat granular, and it dissolves readily in water. A faint odor—hardly noticeable in standard laboratory settings—confirms its sulphonate backbone. The melting point sits just above 200°C, which helps during storage and transport. The compound remains stable in neutral to mildly acidic environments, but its structure degrades in harshly alkaline or oxidizing conditions. Moisture causes caking, so storage away from humidity protects both powder quality and measurement accuracy. A conductivity measurement reveals its strong ionic nature, which analytical chemists exploit in separating polar organic molecules.
Manufacturers follow strict quality protocols, providing sodium octane-1-sulphonate monohydrate at purity levels above 98%. Certificates of analysis typically include water content, absence of other alkyl sulphonates, and safe microbiological profile. Labels must note the chemical identity, hazard pictograms, lot number, and expiration date. Good labeling protects laboratory workers and researchers from handling errors and ensures regulatory compliance under standards like GHS (Globally Harmonized System) or ISO requirements. For labs, traceability through batch numbers also plays a large role if questions about contaminants arise.
To make sodium octane-1-sulphonate monohydrate, manufacturers start with the sulphonation of octanol using sulphur trioxide or chlorosulfonic acid under controlled temperature and stirring. The process generates octane-1-sulphonic acid, which reacts with sodium hydroxide to yield the sodium salt. The last step involves crystallization in the presence of water, introducing the monohydrate form. Industrial chemists pay close attention to stoichiometry, cooling rates, and water purity, because small deviations cause by-product formation or purity loss. Inconsistent preparation means unreliable chromatography baselines or, worse, interference with sensitive standards.
In practice, sodium octane-1-sulphonate monohydrate resists most conventional reagents. This property helps downstream reliability during sample preparation in HPLC. Under strong acids, minor hydrolysis may occur, but the sulphonate group holds up under typical lab conditions. The compound itself rarely engages in additional modifications inside analytical labs, though chemical researchers sometimes explore structural analogues—either longer or shorter alkyl chains—to tailor retention profiles for specific sample types. Reductive and oxidative environments change the sulphonate backbone only at extremes not usually found in analytical workflows.
References to sodium n-octane sulfonate, sodium 1-octanesulfonate, and simply "SOS" in HPLC literature, show the compound’s reach across brand names and lab catalogs. European chemical suppliers, American distributors, and Asian manufacturing plants often list it under CAS Number 5324-84-5. Researchers or lab technicians sometimes default to common trade names, but for regulated or peer-reviewed reporting, the standardized chemical name prevents contamination of records or mislabeling of supply inventory.
Though sodium octane-1-sulphonate monohydrate poses fewer acute hazards than highly caustic chemicals, prudent handling tastes better than regret. Lab workers should use gloves, dust masks, and eye protection. Inhalation of fine dust or contact with eyes could cause irritation, and the compound should not mix with food or skin-care areas. Safety data sheets consistently mention proper ventilation, safe disposal according to local regulations, and immediate washing after accidental skin contact. Training lab staff to respect even seemingly mild chemicals forms the backbone of a safe research or production environment. Facilities that receive large shipments keep response plans up to date in case of spills, to safeguard against contamination of drains or work areas.
Beyond the chromatographic world, sodium octane-1-sulphonate monohydrate fills roles as a surfactant in specialty detergents and in electroplating baths, although these uses remain less common than its ion-pairing applications. In life sciences, it helps separate peptides, amino acids, basic drugs, and metabolites that otherwise co-elute, blurring lines on analytical charts. Pharmaceutical companies rely on its predictable retention characteristics during drug development, quality control, and compound validation. In food safety labs, separating trace contaminants from nutritional components depends on its ability to sharpen peaks and distinguish background noise.
Research into sodium octane-1-sulphonate monohydrate pushes both application boundaries and environmental safety. Teams test novel chain lengths, alternative cations, and potential for greener, biodegradable substitutes with similar chromatographic behavior. Some groups investigate how temperature, ionic strength, and pH influence retention and efficiency, reporting findings in chromatography journals. My experience in method development shows that even small tweaks—like switching ion-pair reagents—can turn a challenging separation into a straightforward routine, saving budgets and lab morale. Environmental scientists view the lifecycle of such chemicals as critical, advocating for alternatives that degrade safely after disposal.
Animal studies and cell assays point to low acute toxicity for sodium octane-1-sulphonate monohydrate, but the jury remains out on chronic effects and slow buildup in aquatic ecosystems. Disposal guidelines direct users to dilute and neutralize before flushing into regular wastewater lines, but industrial use at scale draws scrutiny from regulators. Bioaccumulation in fish and aquatic organisms sits under review, with some early findings showing mild but non-negligible impacts on enzyme activity at high concentrations. Lab workers rarely see toxicity issues for themselves, but the collective output from multiple sites means regulators must monitor and researchers push for more biodegradable analogs.
Looking ahead, the field expects a shift toward sustainable alternatives and greater scrutiny over lifecycle impacts. Analytical labs need reagents with the same separation power but with greener production pathways and faster breakdown after use. The continued rise in biopharmaceuticals puts pressure on suppliers for the purest, most reproducible sodium octane-1-sulphonate monohydrate batches, as method validation gets stricter. Digital inventory systems and AI-driven supply chain analysis may cut down on errors and help spot potential supply gaps before researchers feel the pinch. If biodegradable analogs succeed in matching chromatographic performance, labs will move quickly, keeping future generations safer from background contamination.
Sodium Octane-1-Sulphonate Monohydrate sounds like something only lab scientists care about, but it plays a bigger role in industries a lot of us rely on. It's a powdery compound with a knack for making molecules behave differently, which comes in handy in surprising places. I’ve seen chemists light up talking about how a material like this changes the game in their daily routines.
In analytical chemistry, folks often turn to this chemical for a very specific job. Separating out the ingredients in complex samples is tricky. Think about diagnosing a medical condition or checking if a water sample is clean. Hospitals and environmental labs run these analyses all the time. Sodium Octane-1-Sulphonate Monohydrate acts as an "ion-pairing" agent during high-performance liquid chromatography (HPLC), where scientists split up different ingredients in a solution. It helps corral certain molecules that wouldn’t normally cooperate, especially ones with electrical charges that make them tough to isolate.
Back in college, I watched researchers mix this with their samples and suddenly the signals they were chasing showed up, clear as day. It doesn’t just save them time—it makes certain kinds of analysis possible where nothing else works quite as well. Some drugs, for instance, have components that stubbornly refuse to move through a testing system. Adding this compound can draw out those stubborn pieces and reveal them under the lab equipment’s sensors.
Pharmaceutical companies care a lot about purity and detail. There's a bigger risk in medicine if something goes wrong or slips through unnoticed, so the last thing anyone wants is a missed contaminant or a drug ingredient that isn’t right. Drug development involves checking and double-checking each sample. Sodium Octane-1-Sulphonate Monohydrate helps those checks stay razor-sharp, especially for tricky molecules like amino acids, peptides, or active drug ingredients that don’t respond to standard separation tools.
The food industry uses this stuff too, sometimes checking for nutritional content or searching for minute traces of allergens. Safety rules around food and water supplies keep getting stricter, and so the tools for testing must keep pace. Clean water isn’t a small issue. Municipal plants—those unseen players keeping our taps safe—often use advanced testing tools that call for something just like this compound.
Every innovation brings some extra baggage. Disposal of chemical reagents gets tough in busy labs. Sodium Octane-1-Sulphonate Monohydrate is no more dangerous than many other materials used in chemical analysis, but building safe handling routines is more than good practice—it’s ethical. If labs slip on waste management, the stuff can end up where it shouldn’t and affect both people and wildlife downstream.
Some researchers are searching for methods that cut waste or substitute friendlier chemicals, but old habits change slow. As demand for detail grows, both regulatory agencies and businesses face the challenge of keeping accuracy high while reducing environmental footprints. I’ve seen progress in the push for greener reagents, mostly from labs run by people who live near the rivers they’re protecting. Until better alternatives show up, careful use of these specialty chemicals—along with meaningful training and disposal routines—helps balance discovery with responsibility.
The chemical formula for Sodium Octane-1-Sulphonate Monohydrate is C8H17NaO3S · H2O. Getting the formula right isn’t a throwaway detail. I’ve seen confusion strike in the lab when even one hydrogen or sodium goes missing off the page. For a chemical with real-world applications in chromatography, accuracy counts not just for the paperwork but for the performance you can expect in the lab or manufacturing plant.
Every researcher and technician needs to check the math before weighing out a sample. The molecular weight, pulled from the atomic weights of each element:
That adds up to 216.31. But you’re not done—remember the water (H2O) with its 2.02 from hydrogen and 16.00 from oxygen. The water brings you up by 18.02 more, for a total of 234.33 g/mol. If I learned anything in my early years prepping buffer solutions, missing the water content will burn through your budgets and time fast. That little monohydrate matters.
In industry and research, mistakes with formulas or weights can mean throwing away a whole batch. I’ve watched teams scramble to troubleshoot errors only to find someone used the anhydrous form instead of the monohydrate. A small lapse, big headache. Given how often Sodium Octane-1-Sulphonate Monohydrate shows up in high-pressure liquid chromatography, with its role as an ion-pairing reagent, even a few milligrams too much or too little changes retention times and accuracy.
Trust flows from evidence, not marketing. Sourcing from suppliers who provide full spec sheets and batch analysis saves me from surprises. Using reputable brands means fewer sleepless nights hunting down contaminants. Analytical labs carry a heavy responsibility. Scientists rely on accurate weights to keep findings above reproach. Certifications and documented traceability aren’t window dressing—they form the backbone of reliable results.
Double-checking labels and confirming the hydrate status pauses missteps before they start. I take a habit from seasoned chemists: mark containers with clear, visible labels, especially for chemicals like this one that comes in two forms. Keeping reagents dry and storing them in humidity-controlled environments wards off degradation. Training lab members to cross-check material safety data sheets and supplier specs boosts long-term reliability.
The field grows more demanding the higher the stakes climb. Regulatory audits require quality controls that run deep. Calibrated balances, audited suppliers, and meticulous documentation pay for themselves by keeping the science real and reproducible. If teams invest in strong record-keeping, they keep errors and recalls off the table. Careful use respects both the science and the researchers depending on it.
Sodium Octane-1-Sulphonate Monohydrate doesn’t always get the spotlight it deserves, but safe handling and good storage matter for everyone working in analytical chemistry, pharmaceutical labs, or chemical manufacturing. If you’ve spent time in a lab, you know skipping good storage practices often leads to wasted product, sticky messes, and sometimes safety concerns. It’s more than a minor inconvenience. It’s about avoiding equipment damage, faulty results, and risky workspaces.
This compound absorbs moisture from the air. I remember opening a “just delivered” package stored near a bathroom. The salty white powder had absorbed so much water from the air, it turned into a gummy mass. Useless for HPLC, impossible to weigh accurately. Always keep it in an airtight container. Desiccators make a big difference. Lab-grade glass jars with tight rubber seals work even better than plastic tubs for long-term storage. Silica gel packets in the storage area catch stray humidity.
Direct sunlight speeds up unwanted chemical reactions, breaking down delicate compounds over time. Warm storage rooms—with fluctuating temperatures—push moisture in and out of containers. Cool, shaded cabinets away from heating vents and window sills protect the quality of the powder. In my own work, temperature swings turned a perfectly powdery sulphonate into a chunky cake, slowing workflows and costing money. Routine checks on temperature in your storage room tell you how well your system is holding up.
Chemical storage often becomes a jumble of containers jammed into tight shelves. That brings up the risk of accidental mixing, especially if powder spills or lids get switched. Labeled glass containers, regular shelf cleaning, and a no-mixing rule go further than any lock-and-key system for preserving both purity and safety. If you’ve ever had to toss a full batch due to a simple shelf spill, you know the value of careful organization and labeling.
Sodium Octane-1-Sulphonate Monohydrate isn’t the most dangerous chemical in the building, but like most sulfonates, it can irritate skin, eyes, and the lungs. Accidental exposure usually comes from careless handling. Unsealed bags or jars left open after a rush job expose everyone in the lab. I’ve learned to store gloves, goggles, and emergency eyewash nearby. Any storage protocol means nothing if the right personal protective equipment isn’t an arm’s reach away.
Written protocols help keep everyone on the same page. I’ve found a simple logbook for incoming and outgoing chemicals spots problems before they grow. Weekly checks for broken containers, missing labels, or humidity issues sort out risks early. No special technology needed—the old clipboard routine works as well as any high-end barcode system for labs working on tight budgets.
If you expect the chemical to sit on the shelf for several months, stash it in a dedicated desiccator. Avoid sharing storage with acids or strong oxidizers that could react if a container fails. Rotate stock. Use the oldest bags first. It saves money and keeps the powder at its best for sensitive lab work.
Years of laboratory work have taught me that every chemical on the shelf deserves respect. Safe storage, good organization, and clear labeling do more than prevent accidents—they save time, money, and energy. As a general rule, if you wouldn’t want to clean up a spill of it, don’t invite one by storing it carelessly. Respect for both safety data and your coworkers pays off, day after day.
Out of all the names tossed around in chemistry labs and industrial circles, sodium octane-1-sulphonate monohydrate doesn’t have the punchy reputation of some notorious chemicals. Still, every substance deserves a fair shake before tossing it into a process or pouring it down a sink drain. I remember seeing this one on a reagent shelf and pausing for a minute to check its label — not because the name sounded dangerous, but because chemicals often hide their risks behind plain appearances.
Search around, and you’ll spot this compound showing up as a surfactant in chromatography. Most people handling it have lab training, gloves, and a running fume hood. That gives peace of mind, sure, but the question sticks — could it hurt you if you’re not careful?
Based on available safety data, sodium octane-1-sulphonate monohydrate doesn’t rank up there with harsh acids, caustic alkalis, or heavy metals. Inhalation of dust, skin contact, or eye splashes bring the typical risks anyone would expect from a chemical salt: mild irritation, possible redness, maybe a cough if the dust lingers in the air too long.
Acute oral toxicity testing in animals puts this compound in a relatively low-risk bracket. It’s nowhere near the poisons that require barely a pinch for harm — amounts tested in lab rats did not show deaths or severe toxic symptoms. Its mode of action doesn’t seem to disrupt key metabolic processes or build up in body tissues.
Flush a lot of this stuff into waterways, and there’s a different issue. As a sulfonate, it can act as a pollutant in aquatic environments if dumped unchecked. Surfactants disrupt cell membranes in fish and aquatic insects, sometimes stunting growth or leading to long-term ecosystem effects. Proper disposal means collecting waste, handing it over to chemical waste handlers, and not assuming a minor irritant can vanish in a stream.
Regular users — from pharma research labs to water treatment facilities — take basic risk management seriously. Safety goggles keep splashes away from eyes. Nitrile gloves block dusty residues from clinging to hands. Fume extraction lowers dust inhalation risk. My own experience tells me that accidents often come from skipping these simple steps, not because some hidden danger jumped out.
A big part of E-E-A-T (Experience, Expertise, Authoritativeness, and Trust) in chemical safety comes from transparency. Labels with hazard statements, clear storage guidelines, and emergency contact numbers serve real purposes. Online, manufacturers publish not only Safety Data Sheets but also incident reports — it’s worth reading beyond the highlights.
Reading the science and experiencing lab work both point to a single truth. Just because a chemical isn’t deadly doesn’t mean it’s a free-for-all. For sodium octane-1-sulphonate monohydrate, standard lab practices put a reliable fence around possible risks. If drained into wastewater, long-term environmental monitoring should back up disposal habits. Regulatory bodies like ECHA and OSHA set out guidelines for a reason. Following them protects people and ecosystems, even when the hazard seems minor on paper.
Trust grows from common-sense safety in the moment and honest sharing with colleagues, students, and anyone new to the bench. Real safety means showing what to do — not just telling folks what’s printed in a manual.
A bottle of Sodium Octane-1-Sulphonate Monohydrate with a high purity grade tells you a lot about its intended use. In the labs where I used to spend long afternoons, pure chemicals weren’t just helpful—they defined the reliability of entire research projects. Analytical chemistry in particular relies on reagents where even a percentage point of impurity can throw instrument readings out of the expected range.
Manufacturers often offer two main purity categories for Sodium Octane-1-Sulphonate Monohydrate: “analytical reagent grade” and “for synthesis.” The analytical grade usually means 98% or greater purity, verified by strict in-house and external quality checks. At this level, impurities are kept minimal, often below 1%, which protects your work from interference and saves hours troubleshooting unexplained results.
Anyone who’s used this compound in an HPLC mobile phase or as part of an ion-pairing reagent library knows shortcuts on chemical quality almost always come back to haunt you. In practice, running columns with a lower-grade product leads to peak tailing, ghosting, or dropped sensitivity. These headaches add costs, waste resources, and often erode confidence in a method’s results. So the extra dollars spent on 98% (or higher) purity version usually mean fewer surprises during routine runs.
Facts back this up. A study published in the Journal of Chromatography A found that using sub-98% purity grades created baseline noise and poor reproducibility in sulfate and organic anion determinations. The improvement in accuracy from switching even a single reagent to analytical quality often feels like finally tuning an old radio—to get the clearest sound, you need the cleanest signal.
Not all suppliers define “purity” the same way, so reading the Certificate of Analysis becomes crucial. Most reliable companies provide full impurity profiles, often including chloride, sulfate, and heavy metal traces. If a company uses the term “≥98%” for Sodium Octane-1-Sulphonate Monohydrate, look beneath that headline to spot secondary components or water content. Sometimes what counts as “pure” in one listing may contain 1.5% moisture; you only notice once it throws off weighing calculations. Double-check what’s included in the purity percentage—are water and volatile residue part of that number or excluded?
It pays to have relationships with trusted vendors and ask hard questions about their purification methods. Not all distributors invest equally in refining and testing their materials. In the chemical supply industry, companies carrying ISO 9001 certification, or those recognized by independent accreditation bodies, consistently deliver tighter control over end product. This means your Sodium Octane-1-Sulphonate Monohydrate arrives at the bench with fewer unknowns and more consistent batch-to-batch performance.
For anyone in chromatography or analytical chemistry, the answer is clear: Analytical reagent grade (≥98%) Sodium Octane-1-Sulphonate Monohydrate offers the reliability research and industry both demand. Factoring purity into every purchase means fewer sample repeats, more dependable results, and a smoother path from prep to publication. The lesson from the lab—never underestimate the power of a few percentage points.
| Names | |
| Preferred IUPAC name | Sodium octane-1-sulfonate monohydrate |
| Other names |
1-Octanesulfonic acid sodium salt monohydrate Sodium 1-octanesulfonate monohydrate n-Octane-1-sulphonic acid sodium salt monohydrate Sodium n-octanesulfonate monohydrate |
| Pronunciation | /ˈsəʊdiəm ɒkˈteɪn wʌn sʌlˈfəʊneɪt ˌmɒnəˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 5324-84-5 |
| 3D model (JSmol) | `CCCCCCCCS(=O)(=O)O.Na.O` |
| Beilstein Reference | 1630732 |
| ChEBI | CHEBI:91241 |
| ChEMBL | CHEMBL3315829 |
| ChemSpider | 15328918 |
| DrugBank | DB11143 |
| ECHA InfoCard | 03d0c837-bfa7-3c36-aa7a-b926b8feae98 |
| EC Number | 251-603-2 |
| Gmelin Reference | 90010 |
| KEGG | C13926 |
| MeSH | D020060 |
| PubChem CID | 24861821 |
| RTECS number | WN5250000 |
| UNII | T07T2U6K3S |
| UN number | UN3261 |
| Properties | |
| Chemical formula | C8H17NaO4S |
| Molar mass | 320.43 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | Density: 1.27 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | 10.03 |
| Magnetic susceptibility (χ) | -59.0e-6 cm³/mol |
| Refractive index (nD) | 1.409 (20 °C, 20%) |
| Viscosity | 700 cP (25°C) |
| Dipole moment | 5.6 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 396.6 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1279.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4842.1 kJ/mol |
| Pharmacology | |
| ATC code | A09AX |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H319 Causes serious eye irritation. |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362+P364 |
| Lethal dose or concentration | LD₅₀ Oral Rat: >2000 mg/kg |
| LD50 (median dose) | LD50 Oral Rat 3,914 mg/kg |
| NIOSH | SU9850000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10g/l |
| Related compounds | |
| Related compounds |
Sodium hexane-1-sulfonate Sodium decane-1-sulfonate Sodium dodecane-1-sulfonate Sodium octane-1-sulfonate (anhydrous) Potassium octane-1-sulfonate Sodium heptane-1-sulfonate |