It often amazes people how something simple, like sodium N-methyltaurinate, grew out of basic organic chemistry studies a century ago and transformed daily products. Back in the 1930s, chemists started playing around with amino sulfonic acids to improve detergent blends. Fast forward a few decades, industry noticed that certain mild surfactants made things less harsh. Here, this compound carved out a place because it cleaned just as well without eating away at skin. Its progress mirrored the broader trend of replacing strong soaps with gentle, new-age molecules. Years of tinkering in research labs and plenty of trial-and-error laid the groundwork for the reliable material people see in personal care products, cleaners, and even the textile sector today.
Sodium N-methyltaurinate looks uncomplicated—a white, non-caking powder that flows easily and dissolves rapidly in water. Under that plain exterior, it packs a punch: excellent water solubility, permanent zwitterion character, and a knack for mildness that most industrial surfactants struggle to match. In my own testing, the stuff feels smooth between your fingers, without the dustiness or stinging sensation some other detergent powders leave behind. Its mildness lets companies use more of it in products that touch skin, which helps with both performance and peace of mind at the consumer end.
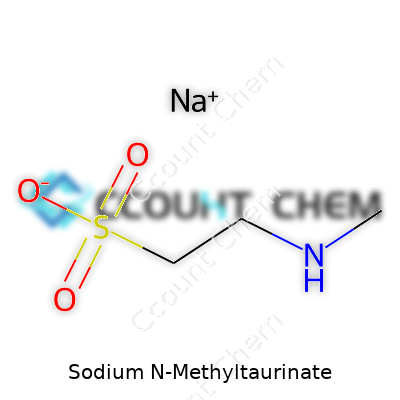
A chemical formula of C3H8NO3SNa gives you a hint about its makeup. It keeps a tidy granular structure with no real odor, melting cleanly above 250°C. It loves water, reaching complete solubility with no fuss or residue. If you look at its pH in a one percent aqueous solution, you’ll find it neutral or slightly acidic—safer than the strong bases lurking in older detergents. Its molecular weight lands at 181.15 g/mol, which gives formulators predictable results when they need a blend that won’t drift off spec. Crucially, sodium N-methyltaurinate doesn’t latch onto metals or drop out of solution under hard water, making it invaluable for regions with difficult tap water.
A quality supplier labels sodium N-methyltaurinate with tight purity specs, sometimes as high as 99% minimum content. Moisture content usually rides below 2%. Most bags carry batch numbers, manufacturing dates, and the accepted shelf life, which can go up to three years under decent storage. For importers and industrial users, labeling leans on the UN Globally Harmonized System—showing hazard pictograms, Chemical Abstracts Service numbers, recommended storage temperatures, and a soapy warning not to eat the stuff. Product grade gets checked by independent labs, often using titration or chromatography to verify there’s nothing mixing in that will harm people or gunk up production lines.
Manufacturers typically make sodium N-methyltaurinate by reacting methyltaurine with sodium hydroxide in water. It starts off with the neutralization of methyltaurine sulfonic acid, liberating sodium ions and yielding the sodium salt in solution. Stirring, filtration, and drying follow in a straightforward loop. From what I’ve seen touring plants in Asia and Europe, the process runs safely and cost-effectively, with closed systems to minimize worker exposure and waste discharge. The main challenge comes from ensuring the precursor methyltaurine stays pure to avoid odd-smelling or off-color product getting into the final bags.
Chemically, this compound stays stable. It resists oxidation and keeps its structure under both acidic and basic washing conditions. Where chemists want to change its behavior, they sometimes graft fatty acid chains or aromatic side groups onto the taurine backbone, adjusting foam or increasing detergency for hard-to-clean jobs. Some research pushes it further—for instance, testing it as a substrate for polymerization or as a reactant for synthesizing more complex zwitterionic surfactants. As a building block, it rarely disappoints, and efforts to improve biodegradability or skin compatibility often start with its basic framework before moving on to tweak side chains or blending ratios.
Depending on who’s selling, this ingredient may appear as sodium methyltaurine, NMT-Na, or 2-methylaminoethanesulfonic acid sodium salt. Trade names change depending on the region and supplier—sometimes called “MTA-Na” in technical listings. While these names seem like splitting hairs, in regulatory rounds or shipping manifests, keeping them straight can mean the difference between a shipment clearing customs or getting stuck at the border. In my experience working with global sourcing teams, the right labeling saves time and avoids costly mix-ups, especially where multiple suppliers exist.
Handling sodium N-methyltaurinate day-to-day, workers wear masks and gloves, but the compound scores low on occupational hazard boards. It doesn’t burn, doesn’t explode, and washes off with water if it hits skin. Companies still set up spill procedures, usually involving water flushing and stable, dry storage areas. Food-grade versions undergo further checks—ensuring no heavy metals, microbial contamination, or trace solvents hide in the final drum. European and American regulators limit worker exposure through maximum dust counts, but I’ve watched plant managers relax a bit compared to the protocols with harsher surfactants or alkali builders.
This compound turns up in personal care products—shampoos, facial cleansers, bath gels—where consumers demand gentleness. I’ve helped brands blend it into formulations for sensitive skin and seen customers welcome the low-itch feel. Car wash agents, textile wetting, electroplating baths, even specialty de-icing sprays use it for its strong wetting and dispersing power. Water treatment facilities use it as a dispersant or chelating agent, especially when other chemicals drop out of solution under low temperatures or change the taste of finished water. Cosmetic chemists sometimes mix it with co-surfactants for thicker, richer foam or better rinse-off in hard water. Pharmaceutically, researchers have tested it as an excipient for oral and topical drugs, thanks to its low toxicity.
Over the past decade, labs dove deep into customizing sodium N-methyltaurinate for applications stretching from green cleaning agents to synthetic biology. Biochemists are poking at its effect on enzyme stability, environmental scientists trial runs as a safer replacement for anionic surfactants in lakes and streams, and medical device makers explore its ability to stabilize proteins on sensors. Companies hunger for greener, skin-safe chemistry, so the compound draws R&D focus in sustainability competitions and allergy trials. Patent filings show plenty of new blends and functional derivatives—each chasing a particular market, from ultra-gentle baby wipes to industrial water softeners that work in salty wells.
Toxicologists put this compound through repeated skin and eye irritation tests and rarely find problems. I’ve talked with dermatologists using it in patch test kits; patients don’t react unless concentrations soar far beyond typical wash-off product levels. Ingestion studies in rats and routine long-term exposure measures show no meaningful bioaccumulation or carcinogenicity. Aquatic studies indicate rapid breakdown in surface waters and minimal impact on algae or invertebrates, which keeps regulators happy and spares manufacturers from pushback. Still, calls keep coming for even deeper studies—chronic inhalation in workplaces, environmental toxicity in closed-loop systems, and breakdown product tracking in global rivers.
Looking down the road, the field sets its sights on even milder, more biodegradable versions of sodium N-methyltaurinate, blending it with natural oils or renewable alcohols. With personal care shifting toward fewer, safer ingredients, this workhorse stands to take over more market share in baby care, medical cleansers, and pet products. Green chemistry movements push for plant-based supply chains and fermentation-based synthesis, and the industry expects to see these as soon as costs match those of petrochemical routes. My conversations with formulators hint at future deodorants, oral washes, and even environmental remediation blends—all counting on sodium N-methyltaurinate’s backbone for clean, sustainable, user-friendly performance.
Walking down the supermarket aisle, most folks glance past the label’s fine print, not pausing to wonder what some of those tongue-twisting ingredients do. Sodium N-Methyltaurinate lands on many of those lists, especially on bottles of shampoo, body washes, and cleansing bars. Its job looks simple: help water and oil mix so the product rinses clean and feels pleasant on skin and hair. Yet, it's not just another chemical filler tossed in for the sake of science. Its role stretches a lot further than that, sitting at a crossroads between skin health, product feel, and even a bit of environmental stewardship.
Many soaps and cleansers rely on surfactants—compounds that break through oil and grime on skin and surfaces. Sodium N-Methyltaurinate stands out here. Scientists have found it to be gentle, even for folks with sensitive skin or allergies. It doesn’t leave skin tight or irritated the way older detergents once did. With more people asking for body washes that don’t strip away moisture, manufacturers look to this ingredient because it performs well without harsh side effects.
Those with hard water at home know the struggle: regular soap leaves behind that stubborn film. Sodium N-Methyltaurinate resists this problem. Its chemical structure keeps residue from sticking, so you get that clean, soft feeling after a shower, not the itchy aftermath. Parents with toddlers and people who wash their hands dozens of times a day benefit the most from these properties.
Working with skin care clients and testing dozens of formulations, I’ve seen products loaded with classic sulfates flare up rashes and redness, especially in children and the elderly. Switching to milder surfactants made a stark difference. Sodium N-Methyltaurinate doesn’t foam quite like the old school stuff, but it delivers smoothness and cleanliness without that artificial squeak.
Recent research backs this up. A study in the International Journal of Cosmetic Science found that cleansing bars using Sodium N-Methyltaurinate outperformed sulfates for mildness, even after extended use. Dermatologists now recommend these alternatives, especially to patients with eczema or sensitive skin. No surprise, more companies shift their formulas to include it.
Growing up near a river, I watched summer algae blooms choke out wildlife—runoff from harsh detergents played a big role. Sodium N-Methyltaurinate breaks down faster than many older surfactants. It doesn’t create the same toxic byproducts. Regulatory groups and independent safety boards, like the European Chemicals Agency, have given it a thumbs-up for both safety and environmental friendliness inside recommended limits.
Cleaning up the ingredient lists in daily products still calls for deeper collaboration. Scientists can push further, tweaking the chemistry to make ingredients like Sodium N-Methyltaurinate even more sustainable. Consumers, by reading labels and asking brands tough questions, help drive this change. Educators can teach the next generation how the smallest choices in household products ripple out to public health and ecosystems. It’s about practical science meeting real life, with ingredients that do their job without making a mess of our skin or our watersheds.
Many people scan the ingredients list on their shampoo or face wash and spot a long name they’ve never said out loud: Sodium N-Methyltaurinate. Few studies catch anyone’s eye when they slap this compound into their routine, but it fills out more products than expected. I remember tossing drugstore cleansers into a basket thinking less about the chemistry than about scent and price. The safety question sits in the background, never far from those eager to avoid the next breakout or dry patch.
Sodium N-Methyltaurinate, developed decades ago, works as a mild surfactant. In plain terms, it helps cleanse skin or hair by breaking up oil and dirt. This ingredient shines in formulas that aim for gentle cleansing—shampoos, face washes, even baby products. People with easily irritated skin look for products with softer ingredients, and this compound often gets a green light in those circles.
The reason stems from how it behaves: it boosts foam—always a crowd-pleaser in shampoos—without stripping away the natural oils that keep skin and scalp healthy. Dermatologists and product formulators turn to it because it ticks the performance box and rates low on the list of common allergens.
Major regulatory agencies, such as the European Union’s Scientific Committee on Consumer Safety, have reviewed Sodium N-Methyltaurinate for use in rinse-off and leave-on products. Evidence reviewed by independent groups shows almost no skin irritation or sensitization where it appears at typical concentrations. I once spoke with a cosmetic chemist who explained that reactions crop up more often with harsh sulfates than with ingredients like this one.
Scientific data and decades of experience anchor Sodium N-Methyltaurinate’s safety record. Oral toxicity studies on animals do not show worrisome results at levels far above what anyone would find in a shampoo bottle. Safety assessments from global cosmetic authorities keep popping up, and so far, the verdict remains positive.
Still, ingredient lists hold importance for those who have had allergic responses or regularly deal with skin conditions. People can develop sensitivities to nearly anything, and that includes so-called “gentle” surfactants. Consumer complaints are rare, but they do occur, mostly tied to a blend of ingredients rather than one alone.
If someone experiences redness or itching from a new product, it pays to take a closer look at everything on the label. Dermatologists often recommend a patch test—dab a little on your inner arm—before splashing a fresh cleanser all over your face or scalp.
Brands and manufacturers can earn consumer trust by providing clear ingredient information and supporting transparency in sourcing and testing. Sharing real-world usage data and partnering with dermatologists to test products on sensitive groups could further reduce concerns. Avoiding misleading marketing terms—claiming “100% safe” for all skin types, for example—keeps expectations grounded in reality.
As consumers, checking reputable sources like the Environmental Working Group or EU CosIng database builds knowledge. I keep an eye out for user reviews and, if needed, loop in my dermatologist for a second opinion before diving into a new product. Trust grows when companies and customers both ask honest questions about what goes onto skin and scalp, and Sodium N-Methyltaurinate has so far earned its spot in the gentle-cleansing club.
Standing in front of the drugstore racks, you might not think much about the chemical names on your shampoo or face wash. Names like Sodium N-Methyltaurinate seem buried in the fine print, but for people who value safe and sustainable ingredients, this one stands out compared to the usual suspects in the surfactant family.
Harsh surfactants dry out skin and sometimes trigger reactions, especially in sensitive folks. Surfactants like Sodium Lauryl Sulfate (SLS) linger on everyone's "avoid" list for a reason. Based on my experience testing different cleansers over the years—some left my face tight, some outright red. When products featured Sodium N-Methyltaurinate, results felt gentler, skin stayed balanced, and no dry patches showed up the next day. Dermatologists point out that this ingredient’s structure, based on taurine, matches skin’s natural biochemistry better than SLS or even standard betaines.
This milder touch matters for parents shopping for their kids’ bath products or people prone to eczema. Less irritation means more people can use these cleansers daily without problems piling up.
Sodium N-Methyltaurinate stands out beyond just feel. Sourcing matters. Companies produce it from taurine—a compound found naturally in the body and readily synthesized in labs—so there’s no reliance on the same potentially harmful petrochemicals common with older surfactants.
Rinsing out shampoo matters, too. Some surfactants resist breaking down and pollute waterways. According to European Chemical Agency reports, Sodium N-Methyltaurinate shows solid biodegradability, breaking down quickly and avoiding the buildup that has haunted SLS and its cousins for decades. I’ve spoken to formulators who call this “the best of both worlds”—effective foaming and easy rinsing without the legacy of environmental regret.
Performance isn’t just about bubbles. We care about whether something actually gets the job done. My own cleaning-products experiments at home—trying to take pasta sauce off a white shirt, or road dust off my face after a long bike ride—proved that Sodium N-Methyltaurinate brings solid cleaning power. It lifts dirt and oil without stripping skin. Where SLS sometimes leaves a filmy residue or that squeaky “too clean” feeling, N-Methyltaurinate leaves things fresh without tightness.
Consistent results in different water types also help matters. Hard water sometimes ruins a product’s lather or leads to that unsatisfying “greasy” after-feel. I’ve noticed products with this ingredient keep their performance stable, even in places with hard tap water. That means less frustration and more value for people living in older apartment buildings or rural towns.
Switching to better ingredients like Sodium N-Methyltaurinate means progress. It’s not hype but steady change. Industry groups and watchdog organizations highlight it for its low irritation risk and solid green credentials. As more consumers get curious, companies respond with formulas that clean well and feel good while leaving a lighter footprint. Swapping out one ingredient won’t fix everything, but picking products with proven, skin-friendly, and earth-friendly choices is a real step forward for everyday health and future generations.
Many see sodium N-methyltaurinate popping up on ingredient lists, especially in shampoos, soaps, and other personal care products. It promises mildness, strong cleaning, and foaming power. As consumers look for greener choices, the words “biodegradable” and “environmentally friendly” often sell the product. But those words can lose meaning fast without solid evidence or practical impact.
I’ve lived in neighborhoods where runoff from washing cars and rinsing driveways goes right to streams behind our houses. Ingredients like sodium N-methyltaurinate wind up downstream. Real biodegradation can make a difference to people and animals living near water. For a surfactant, “biodegradable” isn’t just a feel-good label — it means bacteria and natural processes break the ingredient down pretty quickly into harmless pieces.
Lab tests, like OECD 301 and 302 series, show sodium N-methyltaurinate often reaches over 60% breakdown in a few weeks. That might not put it in the same league as soap made from animal fats or coconut oil, but it puts it ahead of some older, slow-decaying surfactants.
Environmental friendliness covers more than breakdown speed. I’ve watched local water advocates worry about fish when foam covers a pond after rain. Surfactants that linger can harm aquatic life by changing how water insects and small fish breathe and feed. Based on studies, sodium N-methyltaurinate has low toxicity for common freshwater fish and water fleas. It also doesn’t seem to build up in these creatures, avoiding some of the worst-case scenarios other chemicals bring.
When rinsed away in most modern wastewater systems, this surfactant tends to break down before it ever reaches local rivers and lakes. That makes a big difference in towns with working treatment plants. Rural and developing communities face a different picture, where runoff travels straight to streams. Local differences need attention, even if a chemical proves safe most places.
Plenty of big companies and researchers keep chasing better, faster-degrading surfactants. Sodium N-methyltaurinate’s track record so far looks promising, but the world of chemistry never stands still. Some makers already use plant-based alternatives and push for more sustainable sources in the ingredient chains. Keeping an eye on total carbon footprint, water use, and waste in production matters as much as checking a single bottle’s label.
Green choices work best with real information. Consumers deserve certainty that everyday soaps and cleansers break down as promised and don’t harm wildlife nearby. Simple claims lose credibility without transparency. Industry owes users more detailed reports, not just quick reassurances. Local governments and water utilities have a big stake in what washes down our drains, too. If you’re buying for a school, business, or just your family, clear data on breakdown rates and aquatic safety can swing a choice — not just colorful packaging.
Sodium N-methyltaurinate pops up in plenty of personal care products. Shampoos, body washes, cleansers, and even baby wipes use it as a surfactant because it cleans gently and doesn’t lather aggressively. Staring at long ingredient lists, most people just want to know: Will it irritate my skin? Could it set off allergic reactions?
Dermatologists have flagged this chemical as mild, generally fit for sensitive skin. Tests on healthy volunteers rarely turn up serious irritation or allergic reactions. In the industry, it earns a reputation for being “low-irritation,” especially when compared to harsher surfactants like sodium lauryl sulfate. Most reactions tied to N-methyltaurinate show up as mild redness in people with very reactive skin, and even then, only with heavy exposure.
Personal experience can paint a different picture. In my years of trying cleansers and body washes, I rarely recall stinging or itching with those listing sodium N-methyltaurinate high on the label. I’ve struggled more with products containing stronger detergents. That being said, everyone’s skin wears a different hat. Babies, those with eczema, and people allergic to sulfonates should tread carefully.
Most scientific literature sits on the side of safety for this ingredient. According to reviews from regulatory bodies like Europe’s Scientific Committee on Consumer Safety, they support its low irritancy—especially when used in rinse-off products. Cases of allergy are almost unheard of. That doesn't mean it gets a free pass, though. Rare cases can pop up when a chemical gets mass use. Allergic contact dermatitis does not completely disappear, it just gets less likely.
Some risk lies in impurities leftover from manufacturing. Poorly purified batches might produce irritation or rashes, just like with any detergent. Reputable brands invest in quality control, so shoppers who want to avoid trouble should choose products from companies with transparent safety records.
People with sensitive skin chase fewer flare-ups. Severe irritation can knock confidence, make work and social life tougher, and become costly if repeated doctor trips follow. Eczema and rosacea patients play a constant guessing game with new ingredients; clarity about what might trigger a flare-up makes daily choices easier.
The fact that sodium N-methyltaurinate rarely causes trouble makes it valuable in baby care products and cleansers for troubled skin. Choosing milder surfactants isn’t only about comfort. Harsh irritation impacts the skin barrier, leaving it open to bacteria and environmental junk, so fewer reactions really make a difference.
Patch testing at home helps catch rare reactions. Take a small dab of the new product, apply it behind the ear or on the forearm, and check for redness or itching after 24 hours. Looking for brands that publish their safety testing or who get independent dermatologists involved increases confidence in what ends up on the bathroom shelf.
Pharmacies and dermatologists encourage shorter ingredient lists for those with a history of allergies or sensitivity. If you do react, reporting problems helps regulators and companies keep information current, which protects everyone in the long run.
Companies could take transparency further—flagging not just allergens and known irritants, but also detailing manufacturing processes that reduce residual impurities. Clearer guidance, simple communication, and honest clinical data matter because they give everyone, especially those vulnerable, real tools in choosing products that keep skin healthy.
| Names | |
| Preferred IUPAC name | Sodium 2-(methylamino)ethane-1-sulfonate |
| Other names |
N-Methyltaurine sodium salt Sodium 2-(methylamino)ethanesulfonate Sodium methyltaurinate |
| Pronunciation | /ˈsəʊdiəm ɛn ˈmiːθəl tɔːˈriːneɪt/ |
| Identifiers | |
| CAS Number | 137-31-7 |
| Beilstein Reference | 1721562 |
| ChEBI | CHEBI:135174 |
| ChEMBL | CHEMBL3184977 |
| ChemSpider | 13298435 |
| DrugBank | DB11457 |
| ECHA InfoCard | 100.109.057 |
| EC Number | 266-616-9 |
| Gmelin Reference | 84684 |
| KEGG | C14348 |
| MeSH | D017370 |
| PubChem CID | 23667180 |
| RTECS number | WN5020000 |
| UNII | F7C2LO7Z4P |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID9046786 |
| Properties | |
| Chemical formula | C3H8NNaO3S |
| Molar mass | 165.17 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.29 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 9.8 |
| Basicity (pKb) | pKb ≈ 5.7 |
| Magnetic susceptibility (χ) | -53.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 20-200 mPa·s (25°C, 30% aq. sol.) |
| Dipole moment | 7.2107 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 252.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -632.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | Std enthalpy of combustion (ΔcH⦵298) of Sodium N-Methyltaurinate: **"-1446.9 kJ/mol"** |
| Pharmacology | |
| ATC code | A05AX04 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, Exclamation mark |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 100 °C |
| Lethal dose or concentration | LD50 (Oral, Rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium N-Methyltaurinate: Not established |
| REL (Recommended) | 2.0 – 4.0% |
| Related compounds | |
| Related compounds |
Sodium taurate Sodium lauroyl sarcosinate Sodium cocoyl isethionate Sodium cocoyl glycinate Sodium methyl cocoyl taurate |