Stories tracing the creation of sodium cumenesulphonate usually lead back to the rapid expansion of the chemical sector in the twentieth century. This compound did not surface as a matter of coincidence—it responded to a real thirst for improving cleaning agents when the world started using synthetic detergents on a wide scale. Chemical companies, often chasing new surfactant boosters, stumbled upon methods to attach sulfonate groups onto cumene, birthing a family of products with sodium cumenesulphonate among them. Before standardization, researchers experimented with various methods, fine-tuning procedures to coax higher yields and fewer byproducts. Over time, the production logistics became more refined, rolling this compound into the spotlight for industrial and household use.
Talk to factories and labs relying on sodium cumenesulphonate, and one theme stands out: they don’t just want a commodity—they want a workhorse. Sodium cumenesulphonate functions as a hydrotrope, which means it breaks down the rigid habits of water and helps previously insoluble substances mix together. This property showed unique promise for cleaning agents that must blend several ingredients smoothly. The compound owes its popularity to a structure that fuses a benzene ring with a sulfonic acid side group, paired off with sodium. With global trade, sodium cumenesulphonate has popped up in supply chains for paints, cleaning solutions, textile auxiliaries, and, sometimes, pharmaceuticals.
Crystals or fine powder—sodium cumenesulphonate doesn’t ever look fancy, though it turns heads among chemical engineers. It typically comes in white or off-white form, and when you touch it, you’ll notice it isn't sticky or greasy. Throw it in water, and you’ll watch it dissolve away quickly. This trait is a big reason for its use in cleaning product formulas. Its melting point usually hovers around 350 °C (decomposes before boiling), and its solution carries a neutral to slightly alkaline character, hinting at its sodium backbone. The compound resists most oxidation reactions, doesn’t evaporate easily, and has a stable shelf life when kept dry.
Labeling standards might not be anyone’s idea of fun, but they shield both people and the environment. Production plants typically specify sodium cumenesulphonate by purity (often >90% active content), sodium ion content, pH in a 10% solution, moisture range, and color. MSDS sheets always highlight its CAS number (28348-53-0), and regulatory bodies look for clarity regarding residual solvents or by-products such as unreacted cumene or sulfonic acid. Packaging calls for moisture-proof bags to stop caking and unwanted hydration, kept tightly sealed to block airborne impurities.
Sodium cumenesulphonate usually starts its journey with cumene, or isopropylbenzene. Chemists add sulfur trioxide or concentrated sulfuric acid, transforming cumene into cumenesulfonic acid. This acid quickly finds sodium hydroxide, and just like that, the sodium salt forms. Filtration, drying under reduced pressure, and milling bring the final touches. This method, evolved over decades, finds favor because it produces few hazardous wastes and allows relatively simple scaling. Equipment built from stainless steel resists corrosion, so installations last without leaking hazardous by-products into groundwater or the workspace.
Sodium cumenesulphonate does not just sit idle—it interacts with other chemicals for better surfactant blends or specialty additives. It can anchor additional groups, such as ethoxylates, when manufacturers want even greater solubility. In the lab, heating can trigger desulfonation, reverting it toward its cumene roots if conditions run too hot. Though not highly reactive outside acidic environments, the product stands up well when mixed in the presence of other detergents, surfactants, and builders. Its sulfonate group resists enzymatic breakdown, which gives it an edge for industrial uses where harsh physical or chemical settings would torch lesser hydrotropes.
Sodium cumenesulphonate wears many names across global markets. In aged catalogues, you will see “sodium isopropylbenzenesulfonate” or “ISPSA.” Sometimes, suppliers put a spin on branding, calling it simply “hydrotrope NC” or linking it to specific process steps in detergent manufacturing. The substance keeps the same core identity even as its synonyms multiply with local languages and trademarks. This consistency smooths out regulatory approvals and ingredient listings for companies operating in multiple regions.
Take a whiff of sodium cumenesulphonate dust, and your nose will tingle—not because it’s truly toxic, but because it’s a fine powder. Industry standards remind workers to use masks to keep the powder from lungs or eyes. Direct contact could give mild skin irritation if left for long, though washing with water almost always clears up the issue. Factories keep eye-wash stations nearby as a rule. Ventilation remains a priority, not because the compound releases fumes, but to prevent air stagnation and dust clouds. Shipment follows local chemical transport laws—sealed, labeled, and documented from loading zone to user. Waste handling follows environmental protection acts, so nobody slips hazardous residues into municipal water networks.
Sodium cumenesulphonate found its stride in cleaning agents—both household and industrial. You’ll spot it boosting surfactants in laundry detergents, heavy-duty degreasers for garages, and floor scrubbers in schools or hospitals. Beyond that, paint formulators admire its ability to emulsify stubborn pigment mixes and keep them from settling out. Textile mills bank on it for dye baths, finding they get bolder colors and fewer fabric rejects. Some pharmacists lean on it to increase the solubility of poorly dissolving drugs. In oilfield operations, this compound aids in slime dispersal and water injection processes. Farmers, too, use it as a dispersant or stabilizer for crop treatment sprays.
Academic labs and product engineers study sodium cumenesulphonate not out of curiosity, but for better products and safer handling. Some work tries to raise its solubility, so future formulations need less hydrotrope for the same effect. Other efforts focus on lowering the energy needed in manufacturing for climate-conscious plants. Toxicologists screen both acute and chronic impacts, searching for ways to curb even the smallest risks. Universities in Asia and Europe focus on “green chemistry”—modifying the production route to sidestep harsh reagents, recover by-products for reuse, and cut back on water waste. Research groups in North America target custom blends for future eco-label cleaners, motivated by consumer demand for clear environmental benefits.
On the question of safety, years of testing point to relatively low acute toxicity. Industry watchdogs still keep tabs on water discharges and seek out bioaccumulation effects. Fish and daphnia exposure studies show little persistent harm in waterways, which supports regulatory clearance for detergent use at current concentrations. Still, chemical manufacturers must always handle the product with gloves and care, as laboratory animals have shown skin and eye irritation at concentrated exposures. Workplace safety rules keep daily human contact low, maintaining a wide safety margin between regular use and problematic doses.
Rising demand for more sustainable cleaning compounds puts a spotlight on sodium cumenesulphonate’s role. Researchers see potential in blending with biobased ingredients or reconstructing its molecular backbone for easier breakdown in the environment. Companies betting on the circular economy think about ways to recycle process water after using this compound, or about recovery systems to recapture by-products. If future regulations tighten around aquatic toxicity or bioaccumulation, innovation in “greener” sulfonate salts could push the next generation forward. As long as consumers value clear, effective, and safe formulations, sodium cumenesulphonate stands to keep evolving on the lab bench and in the market.
Sodium cumenesulphonate often shows up on the ingredient lists of cleaning products, shampoos, and even industrial detergents. This compound helps manufacturers solve problems in mixing, cleaning, and even energy savings—challenges that most people don’t notice but definitely appreciate in their daily routines. When I first came across it during a stint managing a local pool supply shop, I remember thinking of all the effort that goes into making sure products clean well, stay stable, and rinse away quickly.
Anyone who’s tried to dissolve a powder in cold water knows not every ingredient dissolves easily. Sodium cumenesulphonate helps here: it acts as a “hydrotrope,” which means it gets molecules that usually separate to blend together. Think about dish soaps or shampoos that pour clear, bubble up, and rinse off fast—this behind-the-scenes chemical often carries that heavy lifting. Households see brighter dishes and cleaner hands, but there’s more going on inside those bottles than meets the eye.
Factories run on efficiency. Chemicals that go into cleaners or degreasers must keep costs down and work well in big machines. Sodium cumenesulphonate slots in by helping surfactants do their job without making the mixture too thick or sticky. It helps companies make concentrated products, so fewer raw materials and packaging bottles leave the factory. That’s part of why bigger chains and small businesses both trust blends containing this chemical.
It’s also a safer bet compared to some older materials. Sodium cumenesulphonate doesn’t give off strong fumes or dangerous byproducts, cutting down on environmental risks. For workers and families, this signals a shift toward safer chemistry—one with less fuss around handling and disposal.
Modern cleaning relies on getting dirt and oils off surfaces without leaving residue. Kitchen cleaners, laundry detergents, and heavy-duty degreasers all use sodium cumenesulphonate so the product rinses clean while holding onto stubborn grime, oil, or even mineral deposits. It works in both soft and hard water. In my experience watching municipal cleaning crews, solutions with this additive cut the need for second washes and leave less behind after rinsing. This saves time, water, and money.
As more people look for greener and smarter cleaning, the spotlight is on what’s inside each product. Sodium cumenesulphonate has passed tests on skin irritation and aquatic safety under normal use. Regulatory reports and scientific reviews support its spot on the shelf. Still, no ingredient is perfect—future research keeps an eye on long-term environmental impact, especially as cities search for ways to keep water supplies safe and clean.
Some startups aim to develop even milder alternatives or find plant-based routes to similar molecules. Yet, complete swaps take time, testing, and honest evaluations of performance. Reliability matters when families count on cleaner surfaces or factories run twenty-four seven. The takeaway: while sodium cumenesulphonate might not have social media fame, it plays a smart role in everything from sparkling glasses to streamlined industrial systems.
Long chemical names make people uneasy. Take sodium cumenesulphonate. Its name feels more at home in a lab than under my kitchen sink. Still, plenty of ingredient lists show it in detergents, cleaning sprays, and even dish soap. Add in all the scaremongering online, and the big question rolls in: should we worry about this ingredient in our homes?
Sodium cumenesulphonate breaks up oil and grease, allowing soap to rinse everything away. That’s the main reason companies add it to things like all-purpose cleaners and laundry liquids. Besides boosting cleaning power, it also keeps mixtures smooth so you don’t end up with weird clumps in a bottle. Because of this, it’s everywhere in cleaning aisles, although you may catch it hiding on ingredient labels under a slightly different name.
Looking at what we know so far, most health agencies seem to agree: this ingredient behaves pretty gently. Studies from trusted sources, including those reviewed by the Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA), show that sodium cumenesulphonate doesn’t cause big safety problems on its own. Skin and eye irritation sometimes show up if people spill the raw chemical or get high doses, but in actual household products, concentrations stay much lower.
It doesn’t build up in the body. It breaks down in wastewater plants, so it won’t keep piling up across the food chain. Those points keep it from joining the list of “forever chemicals” that raise big red flags, like PFAS substances.
Allergies rarely happen. If someone has very sensitive skin, any detergent can cause redness, but the culprit usually turns out to be fragrance or dye. The chemical itself rarely tops the list in allergy clinic reports.
A label full of mystery ingredients can feel threatening, and that’s not paranoia—it’s self-preservation. From news about glyphosate to lead in water pipes, everyday people have seen what happens when safety is overlooked. But with sodium cumenesulphonate, there's transparency in the data. Over the past few decades, regulators worldwide have scrutinized its use in both commercial and consumer products.
My own approach with household cleaners has always leaned toward the cautious side. I check ingredients, hunt for safety data, and do quick research if something looks unfamiliar. I expect most parents, pet owners, and anyone with allergies do the same. If I see an ingredient that scientists basically agree has a low risk for adults and kids—especially with normal use—then I feel a little better picking that bottle up from the store.
People want to know what’s inside their cleaning products. The best answer comes from clearer labels and honest information from manufacturers. There’s little excuse for hiding behind chemical codes or confusing technical language. Regulators and companies both should stick with plain English summaries and direct safety data links right on product websites.
Anyone feeling uneasy about sodium cumenesulphonate can find many “free-from” options in eco-friendly store aisles. Baking soda, vinegar, and castile soap handle most home cleaning tasks with zero worry about complex additives. But for regular use with mainstream cleaners, current science gives this ingredient a decent bill of health.
Sodium cumenesulphonate tells an interesting story through its physical and chemical behavior. I always picture its molecules as tiny tools, built for a specific job but adaptable in practice. The compound has a white, crystalline appearance, and it dissolves easily in water. This water solubility makes it a versatile choice in many industries, especially cleaning products where ease of dissolution matters. You won’t find strong scents or flashy colors—just a solid, reliable powder or granulate that does the task asked of it.
In real-world terms, stability matters more than lab data points. Sodium cumenesulphonate stays stable under normal storage and usage. It doesn’t break down easily or react in unpredictable ways when stored in typical environments. Mix it with water, and it behaves predictably. Spill a little on your workbench, and you won’t witness a dramatic reaction. That makes handling this substance straightforward for folks in labs or factories. You still have to keep acids and oxidizers away from it, which is standard advice for most chemical compounds. Safety goggles and gloves aren’t optional, but the risk factor isn’t extraordinary compared to other household or industrial chemicals.
Ask anyone working in cleaning or personal care products about sodium cumenesulphonate, and odds are, they’ve dealt with it when tweaking formulas. The molecule acts as a hydrotrope. Basically, it helps mix water with oily or greasy substances. Without this property, many soaps and detergents wouldn’t blend as smoothly. In bathroom cleaning sprays or even some shampoos, sodium cumenesulphonate works behind the scenes, keeping solutions clear and stable. That improved mixing means better performance and fewer headaches for product developers.
Plenty of other chemicals promise similar results, but sodium cumenesulphonate stands out for being less harsh or toxic. Toxicity profiles show it breaks down with less environmental impact than many older industrial surfactants. That helps companies meet environmental regulations without losing performance. As someone who has seen both legacy chemical plants and modern “green” factories, I notice operators prefer products that balance performance with a lighter ecological footprint.
Accidents in chemical plants stick with you. Sodium cumenesulphonate doesn’t drive headlines about hazardous spills, but safe handling still rules the day. Storage in dry, sealed containers away from direct sunlight extends its shelf-life. For disposal, neutralization and wastewater dilution work, though following local environmental rules matters most. Engineers and safety personnel often ask suppliers for better recycling options and greener manufacturing pathways. Here, sodium cumenesulphonate shows promise—biodegradability scores higher compared to many alternatives.
With regulations and environmental expectations tightening, chemists look for ingredients like sodium cumenesulphonate that offer performance without nasty side effects. Supporting evidence from regulatory boards and toxicology databases back up its lower risk profile for both people and the environment. In my experience, teamwork between suppliers, safety experts, and engineers leads to smarter, cleaner product lines. The future points toward more innovation with chemicals like sodium cumenesulphonate because they offer a strong balance of effectiveness, safety, and sustainability.
Storing and handling chemicals like sodium cumenesulphonate requires real attention to health and safety, not just because rules say so, but because missteps can put people and the environment at risk. In many jobs, I’ve watched how shortcuts and sloppy practices—even with substances that seem low risk—can end in costly mistakes. This particular chemical often pops up in cleaning agents or as a solubilizer, so it doesn’t always come with alarms blaring. Still, the basics of care always hold strong.
Moisture always spells trouble for sodium cumenesulphonate. This material pulls in water from the air, which can lead to clumping or quality loss. Inside warehouses, it stores best in sealed containers, away from damp corners or places prone to leaks. The spaces I’ve visited that run smoothly tend to invest in clear labeling and put dry storage at the top of their checklist. Warmth shouldn’t get out of hand either—temperatures above room level may degrade the product or make packages sweat.
Much of the headache in chemical storage starts with poor packaging. Staff should always check for cracked drums or torn bags before accepting a new delivery. I’ve seen first-hand how costly even a small spill can be, not just in wasted product but in clean-up costs and labor hours lost. Choose containers built to last, resistant to corrosion, and keep them tightly sealed between uses. Double-checking seals or drum lids doesn’t take much time, but it stops a cascade of avoidable problems.
Personal safety isn’t just about gloves and goggles, though both matter. Everyone who might handle sodium cumenesulphonate should get real training on what to do if they spill it or get it on their skin. In workplaces with a low incident rate, management makes sure safety data sheets sit somewhere visible, not tucked away. Handy eyewash stations and clear spill instructions create confidence instead of confusion during emergencies.
Even chemicals with a reputation for low hazard demand respect. In confined spaces, fine powders cloud the air and could irritate lungs. Decent ventilation goes a long way in avoiding problems and makes daily tasks more comfortable. I remember one crew who installed local exhausts over their packaging line—complaints dropped, and there were zero cases of dust-related irritation from then on. A quick sweep after each shift stops the slow build-up of residue that could turn slick or sticky.
Disposing of old or unused chemical calls for care. Dumping leftover product straight down the drain can violate local laws and damage water supplies. Most places have designated disposal containers and partnerships with waste handlers. If you’re not sure, calling the supplier always clears up confusion—I’ve found most will offer advice for free, since they want users to stay safe and legal too.
Handling sodium cumenesulphonate safely boils down to good habits, not just checklists. With the right tools, clear training, and a culture that values doing things right the first time, accidents stay rare and quality stays high. That’s the experience I’ve seen and what the experts teach. Safety grows from small, consistent decisions every day on the job.
Sodium cumenesulphonate shows up in a bunch of cleaning products, where it helps get dirt off surfaces. Plenty of folks in my neighborhood know this product under names they don’t even realize. Hidden in dishwasher tabs, heavy-duty degreasers, or even laundry liquids, it works not because it’s extraordinary on its own, but because it boosts the cleaning power of other chemicals. Now, more people are wondering about the real cost to the environment. Does it biodegrade? Can it count as “eco-friendly” in our everyday lives?
Chemists have run a lot of standard tests on sodium cumenesulphonate. Fresh results from industry-accepted experiments (like those the OECD recommends) show that this compound doesn’t break down quickly in natural settings. Biodegradation rates land on the lower side—less than 60% in 28 days—making it tricky to call the ingredient “readily biodegradable.” For context, many folks expect cleaning agents to break down within weeks so rivers, lakes, or the ocean don’t collect them over time.
I remember seeing data sheets produced by chemical suppliers, and they spell it out: aquatic life faces moderate risk if big doses of sodium cumenesulphonate linger in waterways. Fish or algae don’t die off fast, but long-term exposure stresses their systems and can mess up natural cycles. It’s not the most toxic surfactant out there, but I know from experience with greywater recycling that even the less hazardous ones can build up, especially if homes or businesses crank up their detergent use.
Plenty of companies label their products as “safe for the environment” when talking about sodium cumenesulphonate. They focus on how it doesn’t harm humans at normal exposure levels and skips over the breakdown story. In the real world, toxicity alone isn’t the full picture. Biodegradability plays a big role in what actually happens once all that foam and wastewater exits the drain.
Some data points out that sewage treatment plants do catch and process much of this additive, but not all systems perform at the highest level. Smaller towns or rural septic tanks might let it slip through. From looking at European Union chemical safety registries, it’s clear that sodium cumenesulphonate often passes through with minor chemical changes if not handled by advanced wastewater systems. Most people just don’t realize that a single choice at the laundry aisle stacks up over months or years.
Sodium cumenesulphonate doesn’t stack up well compared to ingredients near the top of eco-rankings, like certain short-chain, plant-based surfactants that break down much quicker. That doesn’t make it a villain, but for anyone trying to cut down on their environmental footprint, it pays to check the labels. Household cleaning doesn’t require perfect purity, just practical steps—maybe try products stamped with independent green certification, or even cut back on detergent volume for everyday loads.
Researchers suggest more energy needs to go into alternatives that handle grease just as well but disappear from nature after a short time. Big brands chase those breakthroughs, sometimes quietly, but consumer demand plays a role too. Swapping to better options and speaking up on social media gets companies’ attention faster than old-school petitions ever did. If I’ve learned anything from growing up with a rain barrel system and watching the odd soap bubble float on by, it’s that the small changes do add up.
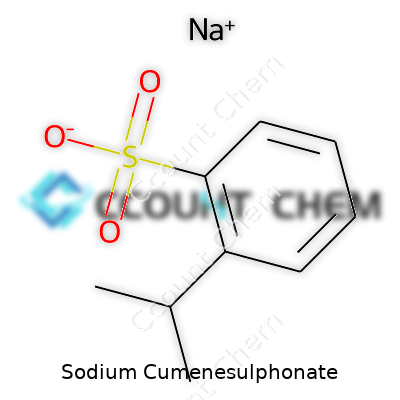
| Names | |
| Preferred IUPAC name | sodium 2-(propan-2-yl)benzenesulfonate |
| Other names |
Sodium cumene sulfonate Sodium isopropylbenzenesulfonate |
| Pronunciation | /ˌsəʊdiəm kjuːˈmiːn sʌlˈfəneɪt/ |
| Identifiers | |
| CAS Number | “28348-53-0” |
| Beilstein Reference | 1898736 |
| ChEBI | CHEBI:91241 |
| ChEMBL | CHEMBL2107798 |
| ChemSpider | 14921 |
| DrugBank | **DB11392** |
| ECHA InfoCard | 100.036.696 |
| EC Number | EC 246-814-9 |
| Gmelin Reference | 77858 |
| KEGG | C01875 |
| MeSH | D020081 |
| PubChem CID | 23665539 |
| RTECS number | GV7350000 |
| UNII | S05D8RFR3A |
| UN number | UN2581 |
| Properties | |
| Chemical formula | C9H11NaO3S |
| Molar mass | 332.40 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 0.6 - 0.7 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.2 |
| Acidity (pKa) | -2.2 |
| Basicity (pKb) | 3.28 |
| Magnetic susceptibility (χ) | -49.5e-6 cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | Viscosity: 10-20 cP (20°C) |
| Dipole moment | 4.4 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 341.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -594.61 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1884.7 kJ/mol |
| Pharmacology | |
| ATC code | Sodium Cumenesulphonate does not have an ATC code |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 100 °C |
| Lethal dose or concentration | LD50 Oral Rat 1300 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 1300 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 260 mg/kg bw |
| Related compounds | |
| Related compounds |
Sodium Xylenesulfonate Sodium Toluene Sulfonate Sodium Dodecylbenzenesulfonate Sodium Benzene Sulfonate |