In the early days of industrial chemistry, the introduction of aromatic sulfonates kicked off a new era for detergents and water treatment. Chemists in the late 19th and early 20th centuries turned to benzene sulfonic acids and their sodium salts because natural soaps weren’t cutting it in hard water. The result was a family of compounds led by sodium benzene sulfonate, offering better solubility, improved stability, and a huge shift away from fats and oils. Early patents from Europe and North America reflect experiments with sulfonation, producing clear records of systematic exploration for additives that don’t break down quickly or promote unwanted foaming. These early choices still shape what we see in today’s surfactant and cleaning product industries.
Sodium benzene sulfonate stands as a crucial intermediate and finished ingredient across industries. It flows out of chemical plants as white, free-flowing powders or granules that dissolve in water like instant coffee. Instead of staying in the lab, it works hard every day in cleaners, dyes, and even pharmaceuticals. Many companies bank on its cheap production and high performance. The backbone of its value comes from a simple structure: a benzene ring with a sulfonic acid group, tied to sodium for stability and readiness to react. This simple formula lets it act as a dispersant, wetting agent, and emulsifier. Big-name factories don’t hesitate to repackage it for a range of uses, so the same bottle might serve textiles, water treatment, and even electroplating operations.
Anyone who has handled sodium benzene sulfonate can spot its white, crystalline look—a sure sign of its purity and straightforward synthesis. Its sharp, slightly bitter taste serves mostly as a warning to keep it away from snacks. The compound easily absorbs water, making it a good fit for solutions but a challenge when you need to keep it dry. It melts only at high temperatures, far above common storage conditions. In water, it breaks apart as sodium cations and sulfonate anions, which help pull apart oil and dirt. Unlike many volatile solvents, it doesn’t evaporate or break down in sunlight, and it avoids chemical drama in storage. Its stability under harsh conditions gives it an edge in applications that eat away at other additives.
Good sodium benzene sulfonate comes with clear paperwork. Most producers post purity levels above 99%, with specific limits on moisture, ash content, and sodium chloride. Industrial users care about these numbers—they translate to performance in real-world situations. Packaging tends toward thick-walled bags or plastic drums, labeled with batch numbers, manufacturing date, and hazard warnings. The compound carries a CAS number (227-357-2 for one common variant) and a chemical formula (C6H5SO3Na). Even the best technical grade material lists trace impurities, so workers know what to expect in the field. Material Safety Data Sheets explain exactly what to do for spills, skin contact, and environmental release.
Making sodium benzene sulfonate doesn’t require rare reagents or exotic equipment. Most plants start with benzene, adding sulfur trioxide or oleum in a controlled reactor—industry veterans call this sulfonation. This step tacks on a sulfonic acid group. Next, neutralization with sodium hydroxide gives the final sodium salt, which then gets dried and milled. More advanced facilities use continuous reactors that cut down on side products and waste, but the basic chemical route hasn’t changed much since the mid-1900s. Larger players invest in emission controls and energy recovery, which started as a cost saver and now stands as a regulatory requirement in many regions. Batch consistency sometimes varies with temperature or raw material impurities, but quality control routines flag out-of-spec material before shipment.
Thanks to its strong acid group and stable aromatic ring, sodium benzene sulfonate holds up to a wide range of transformations. In synthesis labs, chemists modify the benzene ring, either by further substitution with nitro or alkyl groups or via coupling reactions for dye manufacture. In the environmental field, degradation mainly happens through advanced oxidative processes, breaking the aromatic ring and releasing simpler salts and carbon dioxide. The compound’s strong ionic nature makes it a ready partner for ion exchange, which plays out in electroplating or resin regeneration. In surfactant development, structural tweaks change performance: swapping additional alkyl chains or adding ether groups can tailor foaming, wetting, and hydrotropy. Chemical engineers rarely run into trouble with unwanted byproducts after the main sulfonation step, which helps in scale-up for commercial processes.
Walk down any warehouse aisle, and you find sodium benzene sulfonate under different labels: benzenesulfonic acid sodium salt, sodium benzenesulphonate, and in older literature, sulfanilic acid sodium salt. Suppliers love their own codes—SBS, SDBS, or sometimes just “NaBS.” Overseas sources sometimes work under a slightly different spelling or attach trade names that hint at regional market focus, like Sobenate or Benzosol. Buyers and researchers keep a running translation chart to avoid mix-ups, since regulations and customs paperwork care a lot about clear identification.
Operating with sodium benzene sulfonate means more than following a recipe. Workers wear goggles, gloves, and dust masks because the fine powder irritates skin and eyes and brings risk of inhalation. Storage rooms need ventilation and keep humidity low to prevent clumping. Companies enforce training on chemical transfer, spill control, and first aid, since past accidents from dust explosions or careless dumping brought tough lessons. Bulk transport follows DOT, UN, or IMDG codes. Oversight agencies in the US, EU, and Asia set limits for permissible exposure, workplace air quality, and wastewater discharge. Regular checks on facilities and personal exposure prove key in tightening safety performance and minimizing long-term health complaints.
No one can talk about sodium benzene sulfonate without pointing to its wide reach beyond detergents. Water treatment outfits depend on it for removing oil and dirt in municipal and industrial systems. Textile factories count on it as a leveling agent, helping dyes bind evenly to cotton and synthetics. In concrete admixtures, it boosts flow without extra water, making mixes stronger and easier to place. Tanners add it to hides to soften and clean, saving both labor and chemical costs. In pharmaceuticals, it serves as both an excipient and as a component in certain drug formulations, driven by regulatory approval for controlled use. Regulatory filings show hundreds of patents for uses as an intermediate—in pesticides, flame retardants, and corrosion inhibitors. Each field reviews its effectiveness, relying on field performance and ease of disposal.
Big changes in sodium benzene sulfonate applications depend on persistent, targeted research. Academic groups and industrial labs focus on two fronts—earth-friendly production and performance enhancements. One area pockets grant money: recycling benzene derivatives from renewable feedstocks, cutting fossil fuel dependence. Another team works on making biodegradable analogs, test-driving their breakdown in soil and water to keep residues out of rivers and lakes. Computational chemists map out tweaks to the molecule that might curb toxicity or boost biodegradability, feeding new candidates into greenhouse and field trials. A few ongoing collaborations with major detergent makers focus on low-foam formulas for high-efficiency washers, while labs under contract to the oil and gas sector try to push dispersants to handle extreme pH and temperatures. Key papers keep circling back to regulations: future modifications must survive tough new rules on aquatic toxicity and endocrine disruption.
Toxicologists have studied sodium benzene sulfonate for decades, starting with basic assays on rodents and aquatic organisms. Acute toxicity comes low compared to mainstay industrial solvents or pesticides, but chronic exposure through skin or ingestion still raises flags. Long-term studies in fish show that the compound breaks down slowly, which prompted tighter controls on effluent release. Medical research digs into potential skin allergies and rare instances of respiratory irritation, especially among workers dealing with pure powder. Field studies in European rivers connect local concentrations to industrial discharge, prompting investment in closed-loop wastewater systems at chemical plants. Regulators require full lifecycle studies before approving new analogs, which means more funding for lab tissue culture and wildlife monitoring.
Sodium benzene sulfonate will likely see its role stretch as industries demand more sustainable and adaptable chemicals. As environmental standards go higher, companies that invest in green synthesis or bio-based feedstocks will gain an edge over those stuck with old fossil-derived processes. Regulations on microplastics and wastewater discharge get tighter every year, putting pressure on manufacturers to reduce byproducts and packaging waste. On the technical side, compound tweaks aimed at higher temperature tolerance, rapid biodegradation, or broader compatibility with mixed surfactants will become selling points. Research keeps inching toward expanding sodium benzene sulfonate’s reach, especially in advanced materials, eco-friendly cleaners, and pharmaceutical intermediates. Investors who back transparency, clear labeling, and robust safety programs stand to keep their spot in a market that won’t tolerate shortcuts—or outdated chemistry—much longer.
Everyone who’s ever glanced at the back of a cleaning product or a bottle of shampoo might have seen a long list of chemicals. One name that pops up often is sodium benzene sulfonate. Unless you work in a lab, the name doesn’t tell you much. Still, this ingredient quietly plays a big part in making products do their job better.
At its core, sodium benzene sulfonate acts as a surfactant. That means it helps mix oil and water, which never want to blend on their own. In the real world, this shows up in household cleaners that need to pull up greasy messes from kitchen counters or floors. I’ve seen firsthand that a soapy solution with this ingredient lifts off baked-on grime faster than plain hot water ever could.
In shampoos or body washes, this ingredient helps soap lather up rich and thick, spreading it across skin or hair. Anyone who spends time out in a workshop or garden knows how satisfying it feels to wash off real dirt with suds that work. Sodium benzene sulfonate makes that happen by carrying away oil, soil, and sweat and letting water rinse it all down the drain.
Big industries owe a lot to this chemical. Factories use it to keep dyes evenly mixed when coloring fabric, like for sports jerseys or summer dresses. Papermills count on its dispersing ability too, stopping color or resin clumps from forming in paper stock. During my college days working at a print shop, paper treated with ingredients like sodium benzene sulfonate always ran smoother—no jams, no streaks.
Water treatment relies on it, especially for cleaning up municipal water and wastewater. Grease, oil, and other gunky stuff don’t want to let go, but these surfactants force them to break up. By helping water plants clean more efficiently, it helps communities keep healthier and safer tap water.
No chemical comes free of concerns. Surfactants like sodium benzene sulfonate can increase the foaming in rivers or lakes if wastewater isn’t managed. Too much foam can choke off oxygen for fish. Regulations in many countries control how much ends up in waterways. The European Chemicals Agency and U.S. Environmental Protection Agency both call for regular reviews of its use and waste treatment, looking out for both health and water quality. Household products don’t usually use enormous amounts, but in large-scale operations, companies get held to strict safety and waste disposal rules.
People who care about skin and allergies may want to look out for it—especially if they have sensitivities. While sodium benzene sulfonate isn’t widely classified as a top allergen, some skin types don’t love surfactants and can get irritated with frequent exposure. This came up a few times for me and folks in my circle who use lots of hand soap or dish detergent over long shifts.
Transparency from companies helps consumers make better decisions about what goes down their drains. Some brands now offer surfactant alternatives or boast about biodegradable ingredients. Still, without surfactants like sodium benzene sulfonate, cooking oil stains and greasy car parts would stick around a lot longer. It pays to read product labels, ask about disposal, and choose trusted brands that meet real safety standards.
Cleaners, shampoos, textiles, and water plants all benefit from this unassuming powder. Used responsibly, it fits into modern life while letting people balance convenience and care for the environment.
Sodium benzene sulfonate pops up in household products, especially cleaners, soaps, and shampoos. Manufacturers use this ingredient for its ability to help other ingredients mix with water and spread evenly. It’s a kind of surfactant, just like the substances in dish soap that cut through grease.
Research in toxicology journals, including findings by the International Journal of Toxicology, shows sodium benzene sulfonate tends to stick to the surface of the skin rather than penetrating deeply. Studies on human volunteers and laboratory models point out that accidental contact might trigger redness or mild irritation for some people, but not everyone. A review by the WHO reported only temporary redness in a minority of participants using soaps with this chemical, with symptoms fading after usual washing routines.
It’s easy to shrug off chemical names, but anyone who’s ever felt itchy hands after cleaning knows that not all reactions need a science degree to spot. Growing up, my family used multipurpose cleaners—sometimes after using them barehanded, my skin felt tight or chapped. Friends with sensitive skin reported stronger reactions, even a bit of burning. Most people didn’t notice anything. That mix of responses shows why personal care matters just as much as lab data.
Individuals with eczema, psoriasis, or naturally sensitive skin face a higher risk of irritation from any synthetic soap or cleaner, including those with this chemical. A study in Contact Dermatitis linked sodium benzene sulfonate to patch test reactions in a small group of participants with histories of skin allergies. Frequent handwashing with such detergents can strip away oils and leave the skin exposed to more damage.
No widespread medical alert warns against this ingredient for the general public. The Cosmetic Ingredient Review panel gave it a green light for washes and cleansers that rinse off, based on the low risk of lasting effects. The key factor is the amount and the time spent on the skin. Leave-on products carry higher risks, but for items you rinse away in seconds, the risk drops.
Nobody wants to choose between clean dishes and hurting skin. Anyone noticing discomfort should try wearing gloves when cleaning or look for soaps and detergents labeled “fragrance-free” and “for sensitive skin,” since those options often skip harsher chemicals. Applying moisturizer after cleaning stops the dryness that happens when natural oils vanish.
Label reading helps too. Ingredient lists are more than legal fine print—they’re the roadmap to keeping your skin calm. Companies now get regular audits from consumer safety groups and independent labs. Websites like EWG’s Skin Deep database break down risks for each ingredient, giving shoppers new ways to check product safety.
Trust in science, but don’t tune out your body’s own messages. If redness, stinging, or rashes show up after using new cleaning products, stop and make a change. Talking to a dermatologist often gives clear answers, backed by both clinical evidence and practical advice. Manufacturers need to invest in gentler formulas, while customers can vote with their wallets to encourage safer choices.
Sodium benzene sulfonate turns up in detergent formulas and water treatment plants. I’ve watched warehouse teams store it as if it’s just another powder, but this chemical cares about how it’s kept. It comes as a white to off-white granule or powder, and this form makes it easy to move and measure. Its safety profile is solid—non-flammable, with low acute toxicity—but don’t mistake that for an invitation to let your guard down.
You don’t see a chemical reaction until something goes wrong. This is why keeping sodium benzene sulfonate in a cool, dry spot makes sense. In real warehouses, managers look for spots away from water pipes or any humidity-prone corners. Moisture is more than a nuisance; it can cause the powder to cake, making measurement tough and even affecting its performance in industrial uses. I’ve seen moisture ruin entire barrels, turning a free-flowing product into a sluggish lump, which also drives up costs.
Plastic drums and high-density polyethylene bags tend to work well. Metal containers sometimes corrode over time, which risks contamination. A worker I know once tried using steel drums in a pinch and learned quickly that residues don’t just come out with a rinse. I always look for packaging that seals tightly. If you hear about spilled powder in a warehouse, nine times out of ten someone left a lid off or didn’t close a bag right. These accidents make more work, but they also create dust, which can irritate the eyes and, if there’s a lot of it, even the lungs.
Anyone handling storage for chemicals has run into confusing or faded labels. Accurate labeling saves time and stops mistakes. People sometimes overlook the need to store chemicals away from food and incompatible substances. Mixing sodium benzene sulfonate with strong acids can kick off unwanted reactions. I’ve seen labeling protocols save businesses from expensive cleanup, just because someone knew what was in each barrel. Separate storage makes sense, and the smartest teams keep a clear map or use digital inventory systems to keep chemicals apart.
Sodium benzene sulfonate won’t start a fire by itself, but dust in the air might trigger minor irritation or annoy your cleaning crew. I’ve worked in places where regular sweeping and vacuuming kept the air clear. Ventilation goes a long way; in one factory, improving airflow made a real difference in workers' comfort. Spills are cleaned right away, since procrastination can lead to slipping hazards or tracking powder to other parts of the building.
Shelf life depends partly on storage. Under good conditions, the powder stays stable for years, but temperature swings or exposure to sunlight can degrade it faster. Regular checks keep surprises to a minimum. I once saw a few batches stored under skylights—they aged much faster and left the company with a disposal headache.
Proper training goes beyond a safety video. The best-run facilities take time to explain why dry spots matter and why double-checking seals pays off in the long term. A small investment in staff awareness can prevent product loss and, more importantly, keep everyone working safely. I always encourage teams to keep a logbook, since handwritten notes often catch trends in storage issues long before official audits do.
Plenty of cleaning products use ingredients designed to cut through grease and dirt, but some names look straight out of a chemistry textbook. Sodium benzene sulfonate fits the bill. You’ll find it in detergents, shampoos, and even some industrial processes. The molecule is built for tough cleaning, but that sticking power raises questions about its journey down the drain.
Every time I rinse soap or detergent into the sink, the stuff doesn’t simply disappear. Whether we live on a farm or in the city, wastewater travels to treatment plants or sometimes seeps into local waterways. What’s important is to know whether ingredients in those suds will break down safely or linger for years.
Scientists have dug into how sodium benzene sulfonate—especially its linear chain form—holds up outdoors. Linear alkylbenzene sulfonate (LAS), which is closely related, has been around in household products since the 1960s. Over the years, studies confirm that LAS shows a decent rate of biodegradation in environments where bacteria and oxygen run the show. In aerobic conditions, common at many municipal treatment plants, most of it breaks up within a few weeks.
The picture changes in low-oxygen environments like deep mud or underground water. Here the breakdown process slows down or sometimes stalls. For rural areas or cities with weaker wastewater infrastructure, this becomes a problem. Sodium benzene sulfonate may persist longer, raising the risk of it building up in soil and water.
No one wants chemical residues stacking up in drinking water, soil, or the local pond. There’s solid data showing that most household products using sodium benzene sulfonate break down before they cause trouble, at least in places with strong water treatment. According to research published by the American Cleaning Institute, over 95% of LAS can be removed from wastewater through modern treatment.
Still, that small percentage left over becomes a concern if it keeps accumulating. The European Union and U.S. Environmental Protection Agency both classify LAS (and by extension, sodium benzene sulfonate) as “readily biodegradable,” but they still monitor sewage sludge and surface water for persistent surfactants. Fish and aquatic creatures exposed to high levels of these detergents can experience damage to gills and other tissues, emphasizing the need for responsible use.
I make a habit of reading labels and choosing cleaning products labeled as “biodegradable." It may not change the world overnight, but it steers demand toward brands adopting safer formulas. Companies are working on surfactants made from plant-based sources that break down more quickly and completely. Governments can keep pushing for even tighter discharge limits on surfactants and keep funding better wastewater treatment.
Sharing information matters too. Talking with neighbors or school groups about what chemicals go into the water helps the next generation get into the habit of reading labels and thinking about what happens past the drain. If more of us ask questions and push for greener cleaning, small actions add up.
Sodium benzene sulfonate works well for its job. Whether it’s helping clean oil off a garage floor or tackle stains in the laundromat, it’s hard to match its performance. That said, responsibility doesn’t end with a sparkling sink—we all hold a piece of the water cycle in our hands, and small choices stack up to protect the bigger picture.
Walk down the aisle of any grocery store, and somewhere mixed in with cleaning supplies, you’ll stumble on products hiding a name like sodium benzene sulfonate. Chemists know this stuff as a surfactant. It helps water stick to oil and dirt, letting everything wash away smoother. Because of this quality, you’ll spot it in laundry powders, shampoos, dish soaps, and even some industrial cleaners.
Spend long enough around cleaning work and you’ll notice how your skin tells you when it’s had enough. Sodium benzene sulfonate isn’t alone in drying people out, but it’s still a culprit. People who use cleansers containing this chemical for long periods can end up with red, itchy hands. Dermatologists often see cases where folks pick up rashes after regular use. Sometimes the skin cracks, and infection sneaks in. Allergic reactions aren’t common, but they can happen, especially for folks with sensitive skin.
The dust from powdered forms deserves a mention. Inhaling it can make breathing tougher, especially for people with a history of asthma or bronchitis. Chest tightness, coughing, and sneezing show up in reported cases, and there are stories of lung irritation among workers in detergent factories. Reputable institutions—including the US National Institute for Occupational Safety and Health—list sodium benzene sulfonate as an irritant. Short stints around the powder don’t usually cause lasting harm, but regular exposure brings higher risks.
Eyewash stations in chemical plants aren’t just for show. Get sodium benzene sulfonate in your eyes, and the stinging tells you right away. It doesn’t take much to leave redness and discomfort. Larger amounts can lead to swelling or temporary blurring. Most cases clear up after rinsing, but there have been rare reports of chemical burns if the exposure goes untreated.
The temptation to repurpose containers or try home experiments sometimes leads to accidents. Swallowing household products brings a lot of hazards, and detergents with sodium benzene sulfonate are no exception. Poison control data shows symptoms like nausea, vomiting, and stomach cramps show up quickly after ingestion. Higher doses may bring about irritation or burns inside the throat and stomach. Children are most at risk, so keeping cleaning agents out of reach becomes a no-brainer.
Studies on sodium benzene sulfonate’s long-term impact in humans remain a bit thin. So far, large-scale reviews haven’t turned up links to cancer or permanent organ damage from typical use. That’s some relief, but animal studies show that high concentrations can harm aquatic life. Wastewater from factories finds its way to rivers and starts messing with fish and other organisms. The U.S. Environmental Protection Agency already tracks this compound under its aquatic toxicity guidelines.
Ventilation makes a difference, especially in laundry rooms or janitor’s closets. People who wear gloves report fewer skin problems. Reading product labels and following directions—simple steps, but they work. The push for biodegradable cleaners with fewer harsh chemicals keeps growing, and some companies look for gentler alternatives. It’s always wise to treat products with sodium benzene sulfonate as chemicals worth respect. Don’t mix cleaning products, and store them away from kids and pets. If accidental contact happens, rinse with plenty of water and call for help if irritation sticks around.
Everyday exposure rarely turns serious, but knowledge and habits shape safety for families, workers, and local waterways.
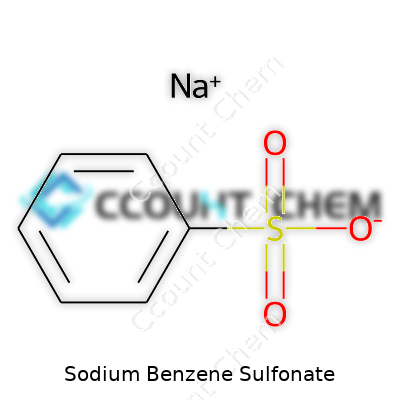
| Names | |
| Preferred IUPAC name | Sodium benzenesulfonate |
| Other names |
Benzenesulfonic acid sodium salt Sodium benzenesulphonate Benzenesulfonate sodium Sodium benzene-1-sulfonate Sodium phenylsulfonate |
| Pronunciation | /ˈsəʊdiəm ˈbɛnziːn sʌlˈfəʊneɪt/ |
| Identifiers | |
| CAS Number | 515-42-4 |
| Beilstein Reference | 1406813 |
| ChEBI | CHEBI:38799 |
| ChEMBL | CHEMBL108770 |
| ChemSpider | 20810 |
| DrugBank | DB15924 |
| ECHA InfoCard | 13eab4c1-6f01-4315-bd13-9802faddb730 |
| EC Number | EC 213-854-3 |
| Gmelin Reference | 82100 |
| KEGG | C01792 |
| MeSH | D014525 |
| PubChem CID | 8651 |
| RTECS number | DJ7175000 |
| UNII | 55X04QC32I |
| UN number | UN2585 |
| Properties | |
| Chemical formula | C6H5SO3Na |
| Molar mass | 288.19 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.485 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.45 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -2.8 |
| Basicity (pKb) | 11.1 |
| Magnetic susceptibility (χ) | -45.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.7 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 356.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -564.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1627 kJ/mol |
| Pharmacology | |
| ATC code | A01AB11 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07; Warning; H315, H319, H335 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Flash point | > 195°C (Closed cup) |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | >2,000 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 400 mg/kg bw |
| Related compounds | |
| Related compounds |
Benzene sulfonic acid Sodium toluenesulfonate Sodium dodecylbenzenesulfonate Potassium benzenesulfonate Calcium benzenesulfonate |