Over the last century, the chemical sector has chased efficiency and cleaner outputs. Sodium 4-Methylbenzenesulfonate, sometimes called Sodium p-Toluenesulfonate, owes its wide adoption to advances in sulfonation. Early 20th-century chemists always looked for ways to introduce sulfonic groups on aromatic rings since this improved solubility and reactivity. During the mid-century boom in synthetic organic chemistry, sodium 4-methylbenzenesulfonate came along as a byproduct in larger projects but found its own niche quickly. Patent filings from the 1950s show manufacturers figuring out how to capture and purify the compound so it could serve as more than just industrial waste. As environmental laws tightened, capturing these “minor” chemicals not only increased profits for chemical plants but also limited pollution.
Sodium 4-Methylbenzenesulfonate stands out as a white crystalline solid, valued for its ability to deliver the sulfonate group in a stable and manageable form. It solves specific problems in synthetic chemistry, supporting the introduction of sulfonic groups to frameworks that otherwise resist substitution. Chemists often consider it not just a building block, but also a valuable additive in various practical procedures, from analytical chemistry to catalysis. Commercial sources list it under several synonyms, including sodium p-toluenesulfonate, and suppliers keep it on hand for both research and production.
On observation, sodium 4-methylbenzenesulfonate appears as a fine white powder or crystalline solid. It shows solid thermal stability, not breaking down easily below 280 °C, and dissolves readily in water. The compound resists volatility, so there's little concern about loss during heated operations unless severe conditions arise. In structure, a sodium ion pairs with a 4-methylbenzenesulfonate anion, bringing an aromatic character from the toluene backbone and strong ionic behavior from the sulfonate and sodium. The melting point usually sits above 300 °C, and the molecular weight lands around 194.19 g/mol. Unlike some organic salts, this one brings little odor and shows no tendency to deliquesce under normal humidity. You see it resist decomposition in both alkaline and most dilute acidic solutions, which keeps it usable in many environments.
Commercial sodium 4-methylbenzenesulfonate typically comes with purity upwards of 98%, sometimes sold as higher grades for sensitive reactions. Industrially packed in moisture-resistant bags or sealed drums, labeling includes hazard classifications, batch IDs, shelf life, and supplier traceability. Certifications from regulatory bodies such as REACH, and documentation of possible residual toluene, support customer assurance. Material safety data sheets lay out handling precautions, disposal guidelines, and recommendations for storage: cool, dry, and ventilated. Laboratories running pharmaceutical syntheses usually keep the analytical data handy—NMR, HPLC profiles, and heavy metal limits—since users rely on predictable reactivity. This transparency reflects broader trends in chemical sourcing and compliance.
Manufacturers start with p-toluenesulfonic acid, itself derived from para-toluenesulfonation of toluene, and neutralize it using sodium carbonate or sodium hydroxide. This neutralization produces the sodium salt directly and leaves behind mostly water and carbon dioxide when using sodium carbonate as the base. Filtering and washing the product cleans away byproducts and unreacted starting material. Older processes sometimes gave inconsistent results, but digitized batch monitoring and better reactor controls let facilities deliver consistent batches every time. Scale-up from bench to plant happens without much trouble—a bonus in a world where time, quality, and cost compete daily.
Chemists reach for sodium 4-methylbenzenesulfonate as both a substrate and a leaving group in organic synthesis. In nucleophilic substitutions, it helps create new bonds where previous routes stalled. The sulfonate group, being electron-withdrawing, pulls on neighboring sites and changes their reactivity. In reductions, it can act as a dummy partner, steering an overall transformation without sticking around in the final molecule. It tolerates many bases and oxidants, and can serve in phase-transfer catalysis as an ionic additive. Hydrogenation and certain coupling reactions reveal the salt’s resilience, where weaker partners would break down or introduce contaminants.
In supplier databases, sodium 4-methylbenzenesulfonate appears as sodium p-toluenesulfonate, sodium toluene-4-sulfonate, and sometimes under trade names chosen by manufacturers. Researchers in biochemistry might simply refer to its shorthand, NaPTS, in lab notebooks. Chemists who work with derivatives or need special solubility features can source custom variants through large distributors, taking care to check CAS numbers and batch documentation for consistency. While synonyms help, quality always circles back to reliable specs and solid technical support.
No one should downplay the importance of safeguarding workers and the environment during routine use of sodium 4-methylbenzenesulfonate. Though considered low-toxicity by regulators, inhalation of dusts or accidental ingestion brings health risks that demand personal protective equipment: gloves, goggles, dust masks, and adequate ventilation top the list. Facilities performing large-scale operations provide spill kits, eyewash stations, and clear signage. Wastewater treatment systems keep residues from escaping and entering waterways. Standard operating procedures require staff training focused not only on immediate hazards, but on cleanup, reporting, and disposal. Auditing aligns with ISO and local chemical safety frameworks, with risk assessments updated as best practices evolve.
Production lines working in dyes, pharmaceuticals, or specialty polymers lean heavily on sodium 4-methylbenzenesulfonate for both mainstream reactions and “trouble spots” other chemicals won’t touch. Its role in efficient sulfonation steps feeds into dye production, giving brighter colors and improved solubility. Drug makers use it as a phase-transfer catalyst, and the salt's compatibility with a range of solvents brings options when designing greener syntheses. In analytical labs, its stable, water-soluble nature aids as a benchmark or internal standard. Electroplaters and surface treaters take advantage of the sulfonate for dispersing agents, especially when metal ions tend to clump. Even the detergent industry sees benefit, using the compound to adjust ionic character in cleaning agents and processing additives.
R&D teams probing greener production methods try to push sodium 4-methylbenzenesulfonate into more catalytic roles, often as a platform for making safer, more biodegradable analogs. The push to minimize waste turns up new hybrid catalysts using the sulfonate moiety as an anchor. Studies in academic labs connect the chemical’s structure with functional outcomes, focusing on reactivity trends, environmental persistence, and the way it changes cross-coupling reactions. Detailed computational modeling now augments bench experimentation, allowing researchers to predict performance and avoid dead ends before investing in full-scale trials. Opportunities emerge around process integration, with the compound poised to help drug discovery, environmental monitoring, and sustainable polymer production.
Toxicologists and regulatory scientists have scrutinized sodium 4-methylbenzenesulfonate for acute and chronic effects. Laboratory animals tolerate moderate doses with little organ damage, and its high water solubility favors low bioaccumulation—a relief in areas with strict wastewater standards. Mutagenicity and carcinogenicity studies have not flagged this compound as high risk, although exposure limits remain in place due to possible irritation of eyes, skin, and lungs from dust. Environmental risk assessments focus on biodegradability, aquatic toxicity, and breakdown pathways, showing that while the sulfonate ion is persistent, the overarching structure degrades under strong treatment. Ongoing research targets subtle ecological impacts and human health exposures in manufacturing zones and end-use sites.
Innovation in the chemical industry often tracks new regulations and the ever-present wish for cleaner processes. With sodium 4-methylbenzenesulfonate, ongoing demand reflects both legacy uses and emerging needs. Advanced catalysis, driven by cleaner energy and materials targets, leverages the distinct sulfonate group for novel reactivities. Consumable goods, from detergents to food contact materials, continue to require additives with strong safety profiles and smart environmental tracking. Circular economy models promote recycling and responsible disposal, keeping the compound relevant as firms look to minimize both production cost and ecological footprint. Academic and industrial partnerships keep targeting more selective, less wasteful syntheses, and sodium 4-methylbenzenesulfonate has room to evolve with these advances.
Sodium 4-methylbenzenesulfonate sounds like a complicated chemistry class term, but in the lab, it pops up more often than folks realize. You’ll hear chemists call it sodium tosylate. Folks in the business of making medicines, dyes, and plastics often lean on this compound for its flexibility. The molecular shape, with its sulfonate group stuck onto a methylated benzene ring, sets it up to play an important role as a stepping-stone chemical. That means it helps move other reactions along, and you see its work show up in finished products without ever seeing it in the ingredient list.
Few people walk into a pharmacy or hardware store asking for sodium 4-methylbenzenesulfonate. Still, manufacturers rely on it for creating building blocks. In organic synthesis, sodium tosylate actually helps other chemicals link up or swap pieces in a controlled way. Think about synthesizing antibiotics or tweaking certain dyes so they’re bright and stable on your shirt after washing. This compound participates in creating what chemists call “leaving groups,” which open pathways to build things that don’t exist in nature. For instance, it lets other groups attach to molecules, making chemical processes cleaner and more precise.
Any discussion about practical chemistry runs headlong into pharmaceutical development. Chemists in pharma lean on sodium 4-methylbenzenesulfonate because it helps transform plain molecules into more complex structures. I’ve learned from researchers that changing a reaction from mediocre yields to success can hinge on using the right helper, and sodium tosylate often proves to be that reliable sidekick. You won’t find this name on a medicine bottle, but it turns up during those millions of tiny steps between a promising idea in the lab and the final drug that gets approved.
The reach goes way past pharma. Creating bright, long-lasting colors in textiles and plastics sometimes depends on the finishing touches that sodium 4-methylbenzenesulfonate can add. Coloring agents or intermediates processed with it stay vivid, resist fading, and don’t bleed as much in the wash. In polymer chemistry, the compound helps with sulfonation, basically letting plastics and resins grab on to new features. This comes in handy for crafts as simple as a tougher drinking cup or as advanced as a water filter membrane. Even in research, students and scientists look to sodium tosylate to test new methods that could lead to lighter materials or more fuel-efficient products.
With any industrial chemical, safe handling goes hand-in-hand with its broad usefulness. Sodium 4-methylbenzenesulfonate typically doesn’t hit the spotlight for public health risks, but those working with it use gloves, ventilation, and strong labeling. Factories and labs strict about chemical safety keep exposures in check and prevent environmental mishaps. Regulatory agencies in many countries monitor its transportation, storage, and disposal—not just for this one substance, but for all like it that flow through the industry.
Looking ahead, researchers keep an eye on efficiency. They search for ways to use chemicals like sodium tosylate with less waste and energy use. Training students and new engineers to respect the risks, follow sensible protocols, and work toward safer alternatives means progress in both science and responsibility. The toolkit of a chemist keeps changing, and sodium 4-methylbenzenesulfonate remains a quiet but crucial piece of modern manufacturing.
Sodium 4-methylbenzenesulfonate comes with a formula: C7H7NaO3S. It looks simple on paper—carbon, hydrogen, sodium, oxygen, sulfur—and yet its chemical behavior goes far beyond a row of letters and numbers. I remember pulling sodium 4-methylbenzenesulfonate from a shelf in the university lab, handling it as a white crystalline powder, reading the label, and realizing, even then, how substances like these sneak into important roles across different fields.
This compound often shows up as a supporting actor in organic synthesis. In my own coursework, it grabbed my attention as an intermediate in making other chemicals, especially dyes and pharmaceuticals. It’s more than a formula—it’s a workhorse, stabilizing reactions and helping chemists isolate cleaner products. Textbooks might skim over real use-cases, but hands-on experience shows that without additives like this, you end up with messy mixtures that stall promising research projects.
People often overlook safety for common compounds. A sodium salt like this, though less hazardous than some of its cousins, still needs the respect any synthetic chemical demands. In any busy lab, proper gloves, lab coat, and an open window matter as much as a careful hand. Years ago, I saw a careless peer rush through weighing a similar sulfonate, sneeze from a fine dust cloud, and then watch as the experiment stalled. Lesson learned: precaution never weighs too heavy, and a clean bench is just as vital as an accurate scale.
Industries rely on such chemical building blocks to make products people use daily. The chemical formula C7H7NaO3S offers more than academic trivia—it’s a passport to a wide range of applications from making colorants that brighten textiles to helping produce medicines that treat disease. Regulatory oversight ensures compounds produced on an industrial scale meet purity and environmental standards. Documented processes help keep polluting byproducts out of the air and water; responsible companies invest in proper containment and waste treatment. It’s not just about ticking boxes—public trust and corporate reputations hang on these practices.
Challenges remain even with well-studied compounds. Many chemists seek greener processes that reduce the need for harsh reagents or make recovery and reuse easier. Students, myself included, run small-scale reactions that still generate plastic waste, energy use, and non-renewable chemistries. Progress happens in fits and starts. Safer laboratory training and access to digital resources make a difference. Teams that communicate clearly about hazards avoid more close calls. In industry, subsidy and policy can push research toward low-impact production and biodegradable alternatives. After all, formulas like C7H7NaO3S don’t work in a vacuum—they need smart, responsible people behind them.
Sodium 4-methylbenzenesulfonate crops up in industrial settings and academic labs, but most folks never hear its name unless they're hunting through chemical safety data. Chemical companies count on it as a reagent. An average consumer has little reason to cross its path. That makes its reputation something of an afterthought, but that doesn't mean risks should get brushed aside. People deserve honest answers about the materials working their way through systems that impact them, directly or indirectly.
Take a look through established records like the European Chemicals Agency (ECHA) or the U.S. National Library of Medicine. They lay out the facts on this compound. Sodium 4-methylbenzenesulfonate doesn’t show up as acutely toxic—no glaring red flags about it being fatal to touch, inhale, or swallow basic amounts. It isn’t listed among substances causing cancer, gene mutations, or reproductive harm.
Lab animal studies offer the main reference point for decision-makers, and reports show low oral and skin toxicity. The eyes can sting if dust floats in, but you’d see that same warning for many basic household cleaners.
Here’s something anyone working with chemicals knows: lack of acute toxicity doesn’t equal a free pass. I've spent years in labs with all kinds of reagents, and the story always goes deeper. Overexposure—think dust floating in the air day after day—carries risks. Even low-toxicity compounds can cause respiratory irritation, headaches, or mild allergic reactions after a long enough run. Gloves, goggles, and good ventilation stop most problems before they start. So while sodium 4-methylbenzenesulfonate seems much less risky than industrial acids or solvents, nobody should treat it like powdered sugar.
Toxicity to humans tells only half the story. Dump enough of any synthetic chemical in the water or soil, and nature could pay a price. The available data show this compound breaking down slowly under some conditions, which nudges regulators to tell companies: collect and neutralize spills, don’t flush solutions down the drain, and make sure waste treatment processes catch what’s left over after production runs. It usually isn’t flagged as highly hazardous, but long-term buildup in one spot hasn’t been studied in depth.
Chemical safety regulations exist for a reason, not just box-checking. As someone who’s seen the results of shortcuts—sick workers, tight-lipped supervisors, piles of paperwork—a few key habits help everyone:
Sodium 4-methylbenzenesulfonate doesn’t top the hazard charts, but information gaps can breed complacency. Routine chemicals in skilled hands cause far fewer problems than ignorance or indifference ever will. Respect, not fear—the balance matters. That’s true on loading docks, in research labs, and anywhere else chemicals push the world forward.
Sodium 4-methylbenzenesulfonate shows up in plenty of research work. I remember using this compound in an organic chemistry lab, mixing it into reaction vessels under the hum of fume hoods. It has a decent shelf life if kept under the right conditions, but I watched one batch go bad after a careless storage choice. Watching weeks of work unravel drives home why storing chemicals the right way isn’t optional—especially for compounds that show up in so many syntheses.
Talking storage boils down to basic science and simple habits. This white, powdery compound won’t spark like sodium metal and lacks the volatility of many organic solvents, but treating it like everyday table salt means trouble. Despite its fairly stable profile, moisture leaks into containers over time in humid rooms. So I’ve learned to keep it in tightly sealed, high-density polyethylene or glass bottles. Polyethylene resists corrosion, and glass won’t leach unwanted substances, keeping the powder clean.
Cool, dry shelves trump corners near steamy sinks or windows, especially in labs without climate control. Temperatures ticking above room temperature accelerate caking and degrade purity. Some folks think desiccators are only for fragile reagents, but I’ve found them just as useful for storing salts like sodium 4-methylbenzenesulfonate. The silica gel inside traps stray moisture, so each scoop stays free-flowing and uncontaminated.
Chemical suppliers echo these guidelines. Sigma-Aldrich and others rate sodium 4-methylbenzenesulfonate as stable under recommended storage, but don’t ignore their safety sheets—the powder still causes eye and skin irritation in careless hands. I keep it far from acids or strong bases. Cross-contamination or a spilled bottle can ruin an experiment or prompt a frantic cleanup.
Labels matter as much as lids. Using sharpies or printed labels to mark open dates and the source batch helps track age and quality changes. Leftovers from old containers sometimes wind up in the wrong flask during late-night runs—that spilled powder never behaves the same way again.
Minimizing environmental impact also means controlling stock levels. It’s tempting to buy big containers to save on costs, but salts forgotten on the back shelf become waste. I measure out small working batches, keeping bulk reserves sealed tight. Most universities and research sites follow strict disposal programs. Pouring salts down the drain, even if they look harmless, puts a hidden load on water treatment plants and nearby rivers. I bundle unused or contaminated sodium 4-methylbenzenesulfonate with other non-hazardous compounds for specialized handling, keeping paperwork tidy to avoid compliance headaches.
Good storage for sodium 4-methylbenzenesulfonate doesn’t start with expensive equipment; it starts with attention to detail—choosing the right bottle, a dry spot off the lab’s main pathways, and always closing lids after use. These choices reflect lessons learned in the field, showing that reliability in chemistry often comes down to how you treat your materials outside the reaction flask as much as inside it.
Understanding the way chemicals behave in water isn’t just for academic curiosity. Picture a laboratory, a wastewater treatment plant, or a pharmaceutical company. Someone grabs a jar marked "Sodium 4-Methylbenzenesulfonate"—a white crystalline powder, another puzzle piece in chemistry’s lineup. Dissolving this compound in water stands as a basic, reliable move in countless labs and factories worldwide. It’s a simple request, yet countless standards, regulations, and product outcomes rely on dissolving this substance completely and predictably.
This compound brings a toluene ring with a sulfonic acid group, making it a sulfonate salt. Water likes ions, and sodium sulfonates tend to give in quickly, losing their structure and scattering through the liquid. Reports from reputable sources such as the Merck Index and peer-reviewed articles state sodium 4-methylbenzenesulfonate has high solubility in water—over 50 grams dissolve in just 100 milliliters at room temperature. That’s generously soluble by everyday lab standards. The molecule’s sodium ion and sulfonate group split and hydrate, ensuring no stubborn granules hang at the bottom of glassware.
Many researchers, myself included, have found humidity and temperature change the way even the most “highly soluble” chemical behaves. During lab work on organic synthesis, this sulfonate salt slid easily into solution. Chemists favor it: it behaves consistently, leaves no residue, and speeds up purification. Water-soluble salts let scientists skip organic solvents, which reduces hazards and simplifies disposal. That translated directly to cleaner waste streams and safer work benches in my own graduate projects.
Pharmaceutical teams rely on that sort of dependability. Any interruptions—like undissolved solids, uneven suspensions, or clogging—delay not just research but the very production of active ingredients for life-saving drugs or antimicrobial treatments. In water treatment, this compound (or its relatives) lands in detergents and flocculants. Consistent dissolution means predictable dosing, which means fewer chemical accidents and better water for everyone downstream.
Even a highly soluble chemical can face hiccups. Cold temperatures, contaminated glassware, or old, clumped material reduce the actual yield that dissolves in water. Over several years of bench work, I’ve seen “high solubility” become “sluggish mess” after samples were stored carelessly or exposed to air for too long, picking up moisture and turning sticky. Basic quality control—dry storage, regular checks on batch purity—keeps that from becoming a bottleneck.
Scale-up from lab to plant brings new headaches. Hard water (with lots of magnesium and calcium) sometimes forces sodium sulfonates to behave unpredictably. A chemist or engineer can test local water first, or opt for deionized water, keeping solubility consistent no matter the location. Automating mixing tanks and using real-time sensors further reduces errors, building in a reliable check if undissolved solids start to appear.
Reliable solubility data saves time and lives. Regulatory agencies trust numbers pulled from decades of careful, repeated experimentation, not marketing claims. Scientists who share their methods, raw data, and even mishaps along the way build a record that future researchers can rely on. When product managers and engineers demand speed, quality, and safety, nobody wants to see a cloud of undissolved powder in what should be a clear, working solution.
Sodium 4-methylbenzenesulfonate solves a simple but critical problem—how to get a consistent, clear solution when dissolved in water. Its generous solubility lets the scientific world rely on it, whether testing new medicines, treating polluted water, or mixing up a batch of research reagents. The story of this simple compound reminds us that what happens in a beaker can have far-reaching impacts, especially if the basics get overlooked.
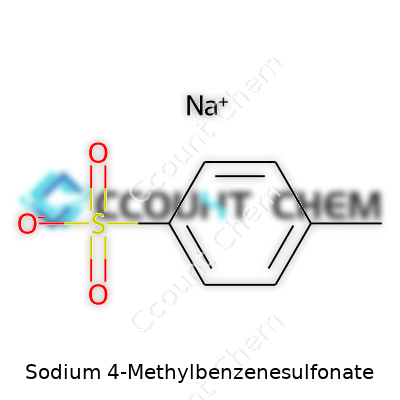
| Names | |
| Preferred IUPAC name | Sodium 4-methylbenzenesulfonate |
| Other names |
Benzenesulfonic acid, 4-methyl-, sodium salt Sodium p-toluenesulfonate p-Toluenesulfonic acid sodium salt Sodium tosylate Sodium 4-methylbenzenesulfonate |
| Pronunciation | /ˈsəʊdiəm fɔːr ˈmɛθəlˈbɛnˌziːnˈsʌl.fə.neɪt/ |
| Identifiers | |
| CAS Number | mes: "657-84-1 |
| 3D model (JSmol) | `CCCC1=CC=C(C=C1)S(=O)(=O)[O-].[Na+]` |
| Beilstein Reference | 1209264 |
| ChEBI | CHEBI:31696 |
| ChEMBL | CHEMBL1373 |
| ChemSpider | 13571 |
| DrugBank | DB14595 |
| ECHA InfoCard | 03b244e8-15bb-4c50-acb1-645c68145567 |
| EC Number | 240-232-0 |
| Gmelin Reference | 103018 |
| KEGG | C16698 |
| MeSH | D014017 |
| PubChem CID | 8663 |
| RTECS number | WJ8925000 |
| UNII | F9T8HN6N8I |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C7H7SO3Na |
| Molar mass | 190.19 g/mol |
| Appearance | white crystalline powder |
| Odor | Odorless |
| Density | 1.33 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -2.8 |
| Basicity (pKb) | 12.0 |
| Magnetic susceptibility (χ) | -34.5e-6 cm³/mol |
| Refractive index (nD) | 1.484 |
| Dipole moment | 3.77 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -831.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1632.2 kJ/mol |
| Pharmacology | |
| ATC code | A01AB21 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS labelling: Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P321, P332+P313, P333+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 210 °C |
| Lethal dose or concentration | LD50 oral (rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): >2000 mg/kg |
| NIOSH | WN5250000 |
| PEL (Permissible) | Not established |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
4-Methylbenzenesulfonic acid Tosyl chloride Sodium benzenesulfonate Potassium 4-methylbenzenesulfonate 4-Methylbenzenesulfonamide |