Chemists looking to expand the toolkit for synthesizing functionalized sulfonate compounds started paying attention to Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate in the late twentieth century, especially as pharmaceutical and specialty chemical industries looked for new building blocks. Interest in this molecule grew as researchers pushed to develop water-soluble, reactive intermediates for advanced organic synthesis. The compound found its place in laboratories where researchers optimized routes for producing pharmaceuticals, surfactants, and polymer additives. As regulatory standards for sulfonate derivatives tightened around the world, especially in major markets like the US and EU, manufacturers invested in better purification processes to meet purity benchmarks, allowing expansion into medical and crop-protection applications.
Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate belongs to a unique cluster of sulfonic acid derivatives, prized for its blend of hydrophilic and electrophilic groups. With a four-carbon chain linking the sulfonate and chloro functionalities, this compound steps into chemical spaces where reactivity toward nucleophiles and water solubility matter. Chemists reach for it as a stepping-stone in synthesizing bioactive molecules, as well as a reagent for introducing sulfonate groups onto complex structures. Labs and factories that handle it often do so in response to contract manufacturing requests from clients in the pharmaceutical, polymer, or agricultural domains, responding to the call for molecules that combine ease of handling, solubility, and reactivity.
Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate comes as a white to off-white, crystalline powder, somewhat hygroscopic and freely soluble in water. The sulfonic acid group (SO3Na) mixes well with polar solvents, while the chloro group delivers potential for further reactions. At typical ambient conditions, the compound remains stable if kept dry and away from strong oxidants or reducing agents. In terms of chemical behavior, the molecules reflect their bifunctional nature: the presence of the good leaving group (chloride) and the charged sulfonate favor alkylation, substitution, and formation of various derivatives. Thermal stability holds through moderate temperatures, although decomposition sets in around 200°C.
Typical commercial grade Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate is specified by minimum assay (usually above 98%) and low limits for chloride, sulfate, and organic impurities. Packages carry detailed batch information, hazard symbols, and recommendations for storage and transport. Material Safety Data Sheets (MSDS) highlight both physical and toxicological properties, noting irritant risks and appropriate first aid responses. Transport regulations fall under non-hazardous status in most jurisdictions, but local rules for sulfonic acid salts apply. Some suppliers secure certification for GMP or ISO standards, responding to the expectations of buyers in life sciences and regulated process environments.
The dominant industrial route uses 4-chlorobutanol as the starting material, which reacts with sodium bisulfite under controlled aqueous conditions. Temperature and pH controls make a difference for both yield and byproduct suppression. As the reaction proceeds, stoichiometric ratios determine whether excess base or oxidants lead to unwanted side chains. Purification usually involves crystallizing the product from water or alcohol/water mixtures, followed by filtration and drying in low-humidity rooms. Manufacturers often run side-reactions at analytical scale to monitor for isomers or side products with different chlorine placements, knowing that these can slip past standard QC if not checked with proper analytical methods like HPLC or NMR.
In chemical synthesis, Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate’s value comes from controlled substitution at the chloro-position, which responds quickly to nucleophiles such as amines, thiols, and phenoxides under gentle conditions. Researchers in the pharmaceutical sector rely on this reactivity to tether sulfonate chains to drug fragments, creating water-soluble derivatives for testing. The sulfonate group remains stable in most basic and slightly acidic media, but strong acids prompt decomposition or desulfonation. Chemists have reported pathways to alkylation, epoxidation (using the hydroxy), and coupling to carboxylic acids, all leveraged in library synthesis protocols. As part of custom synthesis work, manufacturers attach reporter groups or labels to this molecular backbone, making it useful in biochemistry experiments.
In catalogs and patent filings, Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate appears under several alternative names: Sodium 4-chloro-1-hydroxybutane sulfonate, Sodium 4-chloro-4-hydroxybutylsulfonate, and sometimes under trade numbers specific to distributor portfolios. Chemists tracking literature might also see references to its earlier synonyms in Russian, Chinese, or German studies depending on the origin of the research. Proper labeling and cross-referencing with registry numbers (CAS, EC) prevent mix-ups with close analogues in lab ordering systems, a critical step in environments juggling multiple sulfonate compounds.
Responsible use of Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate rests on good laboratory and manufacturing protocols. Even though its acute toxicity runs low compared to alkylating agents, direct skin and eye contact still triggers irritation. Inhalation of dust or solution droplets remains a concern in poorly ventilated areas—this risk demands point extraction or personal respirators when working at scale. Gloves, goggles, and lab coats protect operators during manual transfers. Facilities that use large quantities invest in spill control, emergency eyewashes, as well as smart material labeling to track usage and expiration. Waste solutions need neutralization and dilution to counters risks to watercourses if released in volume—companies taking compliance seriously support discharge permits with routine testing for downstream chloride and sulfonate residues. Training updates and incident drills keep teams ready for unexpected spills or exposures.
Major uses for Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate cluster in pharmaceutical synthesis, specialty surfactant production, and as an intermediate for polymer modification. Drug discovery programs lean on its properties to add hydrophilic properties to candidate molecules, supporting tests for drug solubility and absorption. Surfactant developers choose this ingredient for making new water-dispersible agents, especially where mildness and rapid breakdown in the environment matter. In polymer labs, the sulfonate group attaches to main chain or side chain locations, giving antistatic and dispersing properties to finished products. Smaller but emerging uses include biotechnology, where scientists attach this group to macromolecules to probe new areas in protein solubility and function. Users balancing cost and performance find value in the ready availability of this sulfonated building block compared to sulfonic acids that demand more hazardous manufacturing steps.
Current research centers on broadening the scope of chemical transformations that use this material as starting point. Teams examine new catalysis approaches and green chemistry strategies for substitution and coupling reactions, eager to cut down on waste and boost selectivity. Formulators in pharmaceuticals explore the biological effects of sulfonate-tethered drug candidates, mapping the absorption and metabolic fate of such derivatives in animal models. Some academic groups push the envelope with designer surfactants for oil recovery and pollutant removal, taking advantage of the dual hydrophilic-lipophilic balance the molecule offers. As regulatory climates shift towards greener chemistry, R&D teams test bio-renewable production inputs and new waste treatment regimes for residual sulfonic acids in plant effluent.
Animal studies reviewing Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate place it in the low-to-moderate toxicity range for acute exposure, but repeat dosing draws attention to cumulative irritation and organ load. In vitro assays show low mutagenic or genotoxic potential, yet regulators in Europe and North America keep an eye on long-term environmental exposure due to the slow breakdown of sulfonates. Soil and water monitoring in areas near manufacturing plants drive new regulations on discharge and cleanup. Medical researchers point out rare reports of allergic skin reactions in sensitive individuals, emphasizing the need for comprehensive patient information in pharmaceuticals where the compound or its derivatives remain as trace excipients. Testing of biodegradability across different pH and microbial conditions continues as regulatory agencies raise the bar for environmental acceptability.
I’ve seen growing interest in multi-functional intermediates for medicines and specialty materials—Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate stands out as a candidate for next-generation pharmaceutical platforms, water-soluble polymers, and greener surfactants. Research teams will need to deepen understanding of its breakdown in living systems and the environment to satisfy public health requirements. Digital tools for process monitoring, better solvent recovery, and advances in green chemistry will shape the process improvements. Regulatory agencies, investors, and end-users all have something to gain from collaboration across compliance, R&D, and application adoption. This molecule’s journey has just begun; we can expect new uses, higher standards, and better safety data in the years ahead.
Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate does not attract much attention outside chemistry circles, but it shapes plenty of things most folks use each day. This colorless compound shows up often in industrial labs, sometimes stacked on shelves with other specialty chemicals that support big manufacturing projects. Often, scientists turn to it as a potent intermediate, meaning it joins with other ingredients to build more complex chemical structures. I came across it once in a university lab, working with a graduate team trying to make new surfactants, so its value cannot be dismissed by those who want fresher approaches to cleaning or processing.
Many buyers rely on sodium 4-chloro-1-hydroxybutane-1-sulfonate for producing active pharmaceutical ingredients. The pathway creates molecules with the right kind of chemical “hooks” for antiviral and antifungal drugs. Large-scale factories run reactions using this compound to splice groups onto other base molecules. This makes the medicine more potent, targeted, or easier for the body to absorb. Looking deeper, its sulfonate part helps boost water solubility for the final products of these reactions—a simple thing with hefty payoffs, like getting treatments to dissolve faster for people who cannot wait.
Plenty of material scientists trust this intermediate to pull off specialized sulfonation reactions. These can transform raw chemicals into wetting agents, emulsifiers, and functional additives. New polymers and coatings for electronics or machinery come to life because this chemical provides straightforward ways to alter material surfaces. Working in research, I watched polymer teams juggle a dozen reagents to create just a single new coating—so shaving time off with a compound like this isn’t only nice, it changes budgets and timelines completely.
Chemicals with chlorine atoms and sulfonate groups can raise eyebrows. Some worry about them getting into waterways and building up in wildlife. Manufacturing facilities must set up solid containment, scrubbing, and waste treatment. Health authorities track the use of these intermediates closely—regulations ask for clear documentation, chemical handling plans, and emergency procedures for anyone on site. Over the years, announcements by the EPA and ECHA asked for more frequent audits. Pharmaceutical and specialty chemical manufacturers have to keep looking for ways to recycle or safely neutralize anything left from production. At university, our safety instructor made this clear to every new student—we needed gloves, masks, and written logs for anything sulfonate-based, and violations led to stern warnings.
Researchers keep searching for alternatives where possible, but the unique reactivity window of this chemical makes it tough to swap out for every project. Not every process has a simple, low-impact alternative. Some improvements do exist, like using smaller batch sizes, better purification systems, or stricter emissions testing, all ideas that came up in safety seminars each semester. Change comes slowly but shows up when regulatory pressure rises or when smart folks craft safer, greener synthesis guides.
Anyone who builds new products or medicines needs to watch for new developments in industrial chemistry standards. Fast-moving regulation, solid science, and practical lab skills put limits and possibilities in front of every scientist and engineer. Handling compounds such as sodium 4-chloro-1-hydroxybutane-1-sulfonate calls for teamwork, respect for environmental health, and a big-picture view. In every step, both quality results and safety hang in the balance.
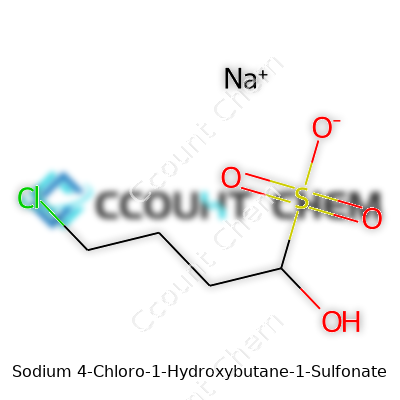
Chemistry names can sound overwhelming until you break them down. Sodium 4-chloro-1-hydroxybutane-1-sulfonate offers a clear story in its name. Look at "butane" and you’ve got a four-carbon chain. Toss in a chlorine atom at the fourth carbon, a hydroxy group at the first, and a sulfonate group also at the first carbon. Then, salt that up with sodium.
Let's put this together step by step. Imagine lining up four carbons. On the first carbon, you’ll find a sulfonate group (-SO3Na) and a hydroxy group (-OH). Head to the fourth carbon, and there’s a chlorine (-Cl) waiting. The backbone looks like this: Cl–CH2–CH2–CH2–C(OH)(SO3Na).
The sodium cation comes from the sulfonate portion. Each group, each position changes how this molecule behaves, both for researchers and for anyone handling it.
In terms of chemical formula, you line up the pieces: C4H8ClNaO4S. That attacks confusion with hard data—four carbons, eight hydrogens, one chlorine, one sodium, four oxygens, and a sulfur atom. Chemists call this systematic, but for the layperson it soon clicks together.
The structure of this compound doesn't just affect its formula. The placement of the hydroxy and sulfonate groups right on the first carbon adds water solubility while the chlorine tacked on the end gives it a bite. This isn’t just an exercise in naming. These groups decide how the molecule breaks apart in water, reacts with other chemicals, or binds to metals.
The way these groups stack up changes real-life use. I’ve spent time in labs where the precise shape and charge of a molecule like this steered reaction outcomes. Compounds like sodium 4-chloro-1-hydroxybutane-1-sulfonate can turn up in processes from industrial synthesis to water treatment. That sodium sulfonate draws in water and fights oil, making the molecule slippery—handy in detergents and cleaners.
Take sulfonates. Water treatment plants rely on sodium sulfonates to grab onto metal ions or oils that shouldn’t head downstream. Hydrophilic sodium groups and oily-slick hydrocarbon chains produce that soaplike action a lot of industries need. Meanwhile, the presence of chlorine can toughen the molecule against microbial breakdown or introduce unique reactivity.
One problem I've watched crop up involves environmental safety. Sulfonates sometimes persist in the water table if they’re not handled right. Their useful properties make them hard for microbes to chew through, which means cleanup needs a plan. Chemists can tweak molecular groups—swapping chains, shifting atoms—or add biodegradable tags to help break these molecules down faster after they finish their job.
It’s not enough to make a molecule easy to use; it needs to leave the environment in good shape, too. Regulatory agencies like the U.S. EPA and agencies across Europe have been sharp-eyed about sulfonate persistence. Making sure the sodium 4-chloro-1-hydroxybutane-1-sulfonate in your process follows best disposal practices isn’t just good sense; it keeps water safer for communities downstream.
Precise structure—a row of carbons, a careful arrangement of sulfonate, hydroxy, and chlorine—shapes everything. It guides function and nudges at safety. Whether you’re elbow-deep in a lab or just hoping for a greener exit at the end of a chemical’s life, every atom on this molecule plays a role.
Anyone who works with chemicals or sensitive products knows that ignoring storage and handling rules can bring real trouble. No matter how familiar a product feels, it never hurts to check the label one more time. Over the years, I’ve seen folks rush storage decisions or skip steps to make space in the warehouse. A few months later, mysterious leaks or product spoilage start popping up, often traced back to simple mistakes made on day one. That’s money and trust straight out the window.
Temperature swings destroy value. If a product label asks for a cool place, that doesn’t mean the back room next to the furnace. It means a reliable spot where temperatures stay steady. I’ve kept thermometers in storage rooms just to catch surprises. For plenty of food, chemical, or pharmaceutical goods, heat and direct sunlight speed up breakdowns or trigger dangerous reactions nobody wants to think about when a client comes to visit.
Avoid letting boxes or containers touch bare concrete floors, especially in damp storage areas. Concrete draws in moisture, which seeps into cardboard and weakens packaging. I’ve seen mold take over entire rows of product just from a week of rainy weather. Pallets give extra breathing room, and regular inspections spot small leaks or odd smells before they become big problems.
Good storage always starts with a clean area. Dust, spilled chemicals, or forgotten cans might seem minor, but contaminants build up day after day. For anything that gets ingested, it only takes one mistake to risk a recall or lose clients who count on safety. Cleaning takes time—it’s easy to push it down the to-do list—but regular schedules for sweeping and wiping down surfaces help everyone on the team spot early warning signs.
It’s natural to think about forklifts and gloves when handling requirements come up, but people form the real backbone. Training makes a bigger difference than new equipment. Most accidents at workplaces I’ve seen started with small “shortcuts” or skipped steps. Regular safety meetings, clearly labeled storage shelves, and making gloves or safety goggles easy to find allow everyone to stay on track. Folks should always double-check for damage before moving anything expensive or fragile—breaking a container in transit means cleanup, paperwork, and more stress for everyone.
The best intentions fade after a busy month. Detailed logs, even simple spreadsheets, keep track of expiration dates, batch numbers, and the last cleaning session. For regulated goods, these logs show inspectors that storage keeps up with the promises made on safety data sheets. I’ve leaned on these logs when a supplier had a recall months down the line. Quick paperwork meant tracking down the affected goods before they made their way out to clients who depend on me.
Investing in proper racks, consistent temperature control, and smart training saves money in the long run. It’s not just spending on fancy tools. It’s about respecting every dollar tied up in inventory and every person who touches these products from start to finish. Storage and handling decisions ripple outward. They leave clear marks on a business’s reputation, client trust, and everyone’s peace of mind at the end of the day.
Sodium 4-chloro-1-hydroxybutane-1-sulfonate rarely shows up in daily conversations, yet it appears in industry and manufacturing. Those who work with specialty chemicals or are around industrial labs know chemicals often get treated as mysterious threats, even though most pose little risk for folks outside a lab or factory. Concerns about health and environmental safety remain crucial, not only for workers but for communities living near production sites.
I’ve spent enough years around chemical plants and talked with plenty of safety engineers. Chemicals usually come with a stack of safety data sheets. Sodium 4-chloro-1-hydroxybutane-1-sulfonate’s sheet notes irritation can happen if enough lands on bare skin or splashes in the eyes. It belongs to a class of sulfonate compounds, which don’t tend to rank high on the acute toxicity scale. The specific substance sometimes gets used as an intermediate, meaning it helps form other compounds, rather than appearing in consumer products on its own.
No evidence points toward high toxicity if you’re looking at casual skin contact or brief exposure. Folks shouldn’t go drinking or inhaling the powder, since, at higher doses, it’s got the potential to irritate lungs and gut, and animal studies around similar chemicals often highlight this type of risk. Anyone who has ever worked with industrial powders knows coughing fits and itchy skin can happen with all sorts of fine dust. Gloves, goggles, and a dust mask sort most of that out.
Serious hazards happen when years stack up working in confined spaces or with poor ventilation. Too many times, industrial hygiene gets overlooked, whether from cost cutting or lack of awareness. Small manufacturing outfits sometimes skip updating their safety procedures. I’ve seen warehouses where people sweep powder off floors with no real protection. Chronic low-level exposure builds up in these cases, which could set off respiratory issues or skin allergies. Stories like these nudge regulators to ask good questions, even for chemicals that start out looking benign.
Another street to consider: runoff and pollution. Much of the public conversation focuses on dumping solvents or acids, but sulfonates still have some water solubility and the capacity to travel through wastewater. Modern waste treatment knocks out most of these chemicals before they hit streams, though accidents and equipment failures can spike levels. Any manufacturing hub with chemical storage tanks risks leaks. That’s less about catastrophes and more about day-to-day vigilance—regular inspections, good spill training, and keeping chemical drums off cracked concrete.
Local and international regulations already demand clear labelling, transport, and disposal protocols for chemicals like sodium 4-chloro-1-hydroxybutane-1-sulfonate. OSHA and REACH set limits not just to keep workers from harm but to shield food and water supplies. Anyone responsible for safety at a site knows just how much paperwork and prodding it can take to keep up with compliance, but these hoops exist because workplace and neighborhood health depend on them.
Folks deserve transparency on what chemicals are near them, whether they work with them directly or live nearby. Risk sits not in how scary a name sounds but in real exposure and the strength of safeguards. Staying updated with new toxicology findings, sharing best practices across industries, and providing ongoing worker training give communities more confidence about what’s actually floating through the air or washing down the drain.
Innovation should push for greener alternatives as soon as research lines them up. In the meantime, speaking plainly about risks and making safety as much a habit as locking the front door will help keep sodium 4-chloro-1-hydroxybutane-1-sulfonate just another tool, not a lurking crisis.
Quality matters, especially for anyone working with chemical compounds. Most buyers look for high purity, often above 99%. Laboratories and production teams rely on these specs to avoid unwanted reactions or skewed results. Pharmaceutical research stands as a good example—any contamination can spoil test results or even derail an entire batch of medicine. While 99% sounds high, even trace residues sometimes create big headaches in sensitive work. That’s why suppliers commonly offer grades above 99.5% for advanced applications. Technical grade options, running high 90s, typically do the trick for industrial uses where a little impurity won’t cause trouble. If you’re sourcing for a regulated field, specifications listed on the certificate of analysis become your benchmark for safety and consistency.
Packaging varies almost as much as customer needs. In a research lab, small glass bottles or plastic vials—usually holding from a few grams up to half a kilo—make storage and handling easier. Higher purity makes contamination control a core concern, especially when opening and closing the container multiple times. For those managing industrial production, larger pails or drums, sometimes up to 200 kilograms, prove much more practical. They drop the per-unit price and cut back on packaging waste. Some suppliers even offer intermediate sizes: 5, 10, or 25 kg sealed containers for mid-sized jobs. If moisture could spoil the material, you’ll see foil-lined or double-walled bags as a safeguard. Solid compounds might arrive vacuum-sealed to protect from air and humidity.
Quality control often starts in the supply chain. A batch with inconsistent purity or poor packaging can ruin a week’s worth of research or production. In my old role at a small biotech firm, a supplier switched from sturdy amber glass to thin plastic bottles without asking. The softer packaging got dented in shipping, and half the bottles already showed contamination when they arrived. We lost two days and had to rework our entire process. Checking lot consistency, trace impurity profiles, and how materials handle temperature change goes a long way. Everything from shelf life to regulatory approval depends on these details.
Building a relationship with suppliers takes guesswork out of ordering. Companies who clearly document their quality control process save their buyers a lot of chaos. Certificates of analysis, batch tracking, and shelf-life information should come standard. Asking about packaging security before signing a purchase order helps prevent surprises and lost money. Buyers in regulated fields sometimes schedule site visits or request stability data for packaging during product launches.
The best safeguard still comes from talking with both technical support and logistics at the supplier. One good question: has this packaging worked for your other clients in a similar field? A little groundwork up front beats scrambling to find a workaround down the road.
At the end of the day, purity and packaging define the real value of a chemical compound. Paying attention to these details offers protection for any project, large or small, and earns trust from partners and customers alike.
| Names | |
| Preferred IUPAC name | sodium 4-chloro-4-sulfonatobutan-1-ol |
| Other names |
Sodium 4-chloro-1-hydroxybutanesulfonate 4-Chloro-1-hydroxybutane-1-sulfonic acid sodium salt Sodium 4-chloro-4-hydroxybutane-1-sulfonate Sodium 4-chloro-1-hydroxybutanesulphonate |
| Pronunciation | /ˈsəʊdiəm fɔːr ˈklɔːrəʊ wʌn haɪˈdrɒksi bjuːˈteɪn wʌn sʌlˈfəʊneɪt/ |
| Identifiers | |
| CAS Number | [29549-49-9] |
| 3D model (JSmol) | `JSmol.loadInline("data/mol;Sodium 4-Chloro-1-Hydroxybutane-1-Sulfonate;NC1=CC=C(C=C1)S(=O)(=O)O.[Na+]")` |
| Beilstein Reference | 1109377 |
| ChEBI | CHEBI:135403 |
| ChEMBL | CHEMBL4220574 |
| ChemSpider | 21869515 |
| DrugBank | DB09439 |
| ECHA InfoCard | 17f3de4b-879d-4bc3-891d-e918527daafb |
| EC Number | 262-898-0 |
| Gmelin Reference | 82235 |
| KEGG | C05791 |
| MeSH | D017366 |
| PubChem CID | 23930444 |
| RTECS number | WO5950000 |
| UNII | Q4K6D0S2V0 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C4H8ClNaO4S |
| Molar mass | 234.63 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.51 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.3 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | -0.7 |
| Basicity (pKb) | pKb > 14 |
| Magnetic susceptibility (χ) | -59.6·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.480 |
| Dipole moment | 4.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 314.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1096.6 kJ/mol |
| Pharmacology | |
| ATC code | V03AB25 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P330, P337+P313, P501 |
| Flash point | > 122.7°C |
| Lethal dose or concentration | LD50 (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): >2000 mg/kg |
| NIOSH | WN0115000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m^3 |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
1-Chloro-4-hydroxybutane 4-Chlorobutane-1-sulfonic acid, sodium salt Sodium 1-hydroxybutane-1-sulfonate Sodium 4-chlorobutane-1-sulfonate 4-Chloro-1-butanol Sodium 1-chlorobutane-1-sulfonate |