Chemistry has a way of unearthing compounds that shift how colorants fit into manufacturing and science labs. Through the late 20th century, as European dye industries sped ahead with organic compounds, the benzene sulfonate family attracted attention for offering bright shades and water solubility with improved stability. The story of sodium 4-(4-chloro-6-(N-ethylanilino)-1,3,5-triazin-2-ylamino)-2-(1-(2-chlorophenyl)-5-hydroxy-3-methyl-1H-pyrazol-4-ylazo)benzenesulfonate follows this path: labs searched for dyes buzzing with strong chromophores, good wash-fastness, and easier dispersal in textile and printing industries. This one grew out of bold steps in triazine ring research, which married colorfast pigment structures and new sulfonation methods that changed how mills processed synthetic fibers.
In simple terms, this dye’s structure hosts two big features: the triazine ring that lets it bond tightly to stuff like cotton, and the azo bridge that fires up vivid coloration. It’s known in industry for bringing a high degree of brightness, good acid resistance, and consistent performance during heat exposure. Color chemists appreciate its solubility in water and even more in alkaline solutions, which fits right into textile and paper processing. Strict quality benchmarks in Europe, North America, and Asia have shaped how manufacturers standardize the purity, tint strength, and dusting tendencies of the material.
This compound appears as a deep orange or reddish powder, based on its batch synthesis. Its molecular weight hovers around 695 g/mol, with a melting point above 230°C, so it stands up to high-temperature dyeing cycles without breaking down. It dissolves briskly in water, builds up a strong absorption in the visible spectrum, and stays stable under ordinary warehouse lighting. Triazine-based dyes sometimes put up a fight against reducing agents, but this structure keeps its color in alkaline and mildly acidic baths. Real-world batches land between pH 8-10 and do not clump if stored away from moisture.
Buyers expect labels showing purity content, tinting strength, water content, and thorough heavy-metal screening. Packaging usually comes in moisture-proof bags or fiber drums, clearly marked with hazard and handling icons, GHS-compliant pictograms, and a CAS number for regulatory reviews. Responsible producers provide full lot testing showing minimal salt content and absence of banned aromatic amines, which keeps the product in line with modern European REACH rules and California’s Prop 65 framework.
Synthesis starts with sulfonating an appropriate chlorobenzene precursor, adding steps to build the pyrazolyl and azo linkages using diazotization and coupling with triazine-type amines. The process steers clear of over-chlorinating and controls color by adjusting pH and salt addition during coupling. Purified dyes run through filtration and drying, then finished with sodium conversion for water solubility. Reliable manufacturers run elaborate QC tests on every batch—spectroscopic checks, pH, solubility, purity against known calibration standards, and bulk density.
The triazine ring lets the dye connect to cellulosic material under alkaline settings, where cotton fibers can form tight covalent bonds with the dye. Its azo group, featuring a -N=N- bridge, drives the charge-transfer that delivers rich colors. Many custom blends swap out the N-ethylanilino group for longer or bulkier chains, aiming to shift solubility or tweak the final shade. Intermediates sometimes serve as linkers or anchor points for further functionalizations in colorant R&D.
Industry catalogs show a handful of registered names, often coded by the Color Index or local manufacturers’ schemes. Synonyms can include variants like Reactive Orange XXX or specific manufacturer model numbers, designed for internal tracking and import/export filings. Labs in different regions have coined names tied to their proprietary synthesis or blending methods, but the long chemical IUPAC name remains foundational for patent filings and regulatory documents.
Standard SDS documents warn users to avoid inhalation or dust contact with skin. Personal experience in a pigment lab hammered home the need for gloves, masks, and well-ventilated stations; even trace amounts can cause mild dermatitis, and chronic exposure ought to be avoided. Facilities install dust extractors and mandate batch logs for chemical handling, since spot checks by health and safety officers are par for the course in major manufacturing hubs. Spills must be swept up, never blasted away with hoses, to limit run-off into wastewater streams.
The major use remains in textiles: printing, direct dyeing, and as a component in reactive dye blends for boosted wash and rub fastness. Paper plants use similar dye structures for sustained tinting of specialty grades, such as high-visibility forms and decorative wraps. Ink makers squeeze value out of its consistent hue and easy dispersal in aqueous or low-VOC systems. Sometimes, specialty chemical companies use the intermediate as a stepping-stone for research into molecular sensors or biological stains, given the dye’s vibrant color and responsiveness in test tubes.
R&D circles focus on keeping up with environmental and consumer health rules, so projects often research how to substitute safer raw materials or create derivatives that break down faster under sunlight or bacteria. My time in industrial color chemistry saw constant study of fastness rates, migration in synthetic fibers, and side-by-side trials with more eco-friendly alternatives. Researchers track how slight tweaks—adding a methyl or ether group—change the finished dye’s performance on modern fibers or recycled cellulose. Often, companies sponsor studies alongside universities to stretch applications to new synthetic blends or specialty technical papers.
Azo and triazine dyes sometimes get flagged for safety testing, since a few members of these chemical classes have produced concerning results on long-term toxicity screens. Regulators push manufacturers to prove the absence of carcinogenic amines and to present full toxicity portfolios—oral, dermal, and aquatic—especially for dyes that may wind up in consumer items close to skin. Long-term mouse and fish model studies do not show serious bioaccumulation or rapid breakdown into dangerous components for this compound, but countries update hazard labeling as more data trickles in.
Future growth for this dye class tracks shifts in textile tech and regulatory climates. Green chemistry will keep pushing for routes that cut waste and use milder reagents, while recyclers look for colorants that don’t stubbornly hang around in worn textiles. With the demand for safe, reliable coloring agents showing no sign of fading, researchers and dye houses hunt for new analogues sharing this structure’s bonding strength while ramping down risks to humans and aquatic life. The best route forward links chemistry savvy with transparency and ongoing risk review, adapting fast to what science discovers and what lawmakers say is right for everyone’s safety.
I remember the first time I stumbled across the name Sodium 4-(4-Chloro-6-(N-Ethylanilino)-1,3,5-Triazin-2-Ylamino)-2-(1-(2-Chlorophenyl)-5-Hydroxy-3-Methyl-1H-Pyrazol-4-Ylazo)Benzenesulfonate. Nobody uses the full name in conversation—chemists included. People working in manufacturing or quality control just call it CI Reactive Red 195, a synthetic dye with deep roots in the textile industry. Factories producing everything from plain cotton t-shirts to vivid patterned scarves have relied on this specific compound for its ability to create lasting, vibrant colors. The name itself hints at the chemical complexity behind colors most people take for granted.
For years, synthetic dyes like this one changed the way businesses produce colored fabrics. Long before synthetic dyes, dyers stuck to plant extracts and animal-based colorants. The results faded quickly under sunlight and multiple washes, often turning clothes dull and lifeless. What this dye delivers is a reliable burst of intense red or maroon that sticks to fibers and resists washing and sunlight. Brands can keep producing the same bright shade of red season after season, without worrying about different dye lots turning out differently. It’s not just fashion, either. Yarn, rugs, towels, and even medical textiles benefit from consistent coloring.
Using this dye carries responsibility. Most folks forget that big chemical names come with big implications for health and the environment. Textile workers have reported skin irritation and respiratory troubles if they work without the right protective gear. Grabbing a handful of dyed cloth straight from the mill leaves a fine red dust on the hands if safety protocols aren’t followed. In regions with little regulation, workers can wind up exposed to the raw dye.
Waterways suffer, too. In some countries, factories dump leftover dye into rivers, turning them unnatural hues and harming aquatic life. According to studies published in the journal Environmental Pollution, azo dyes make up about 60-70% of the textile dye market and contribute significantly to water contamination. Azo group breakdown can release hazardous aromatic amines, many of which threaten ecosystems and increase cancer risks for humans living downstream. I’ve seen rivers in dye-producing regions, and the impact isn’t subtle—a rainbow of colors swirls away from factory outlets.
Reducing the harm tied to this dye isn’t rocket science, but it’s also not happening fast enough. Manufacturers have already started pushing for improved wastewater treatment facilities. Effective treatment methods like adsorption, advanced oxidation, and biological remediation help cut the amount of dye entering local water sources. Investments in safer, closed-loop water systems pay off both financially and environmentally.
Switching to safer dyes remains a big conversation in the sector. Some companies search for biodegradable options or insist on cleaner chemistry during manufacturing. It’s not always commercially viable yet, but momentum builds as buyers and regulators apply pressure. Consumers can keep an eye out for certifications like OEKO-TEX, which restrict the use of harmful substances.
Education also matters. Textile workers need training on protective equipment and safe handling. Governments have the muscle to demand accountability—inspections, limits on chemical discharge, and financial consequences for polluters force companies to rethink outdated processes.
Nobody expects shoppers to memorize chemical names. Still, every bright red dyed fabric traces back through a complex web of chemistry, industry decisions, worker health, and the fate of local waterways. Cleaner processes, worker safety policies, and tougher enforcement together start to chip away at the problems that have dogged textile production for decades.
People notice ingredients in everyday products far more than they used to. Flip over a shampoo bottle or check out a snack label—long lists of compounds fill the space. If you can pronounce them all, you’re in the minority. One compound pops up in conversations about safety: the one you’ve never heard of, but find everywhere. Suddenly, what looked like a harmless household product feels like a chemistry experiment.
One thing I’ve learned over years of reading up on consumer safety—trust in a label only goes so far. Most people believe if a compound makes it into their hand soap, it surely passed tough safety testing. The truth isn’t always so comforting. In the United States, thousands of chemicals hit the market every year. The Food and Drug Administration and the Environmental Protection Agency do important work, but industry often outpaces regulators. Ingredients sometimes appear in products before their long-term consequences get sorted out.
A good example comes from my own kitchen. I once used a nonstick frying pan I bought online. After a year, I saw news about certain nonstick chemicals called PFAS—linked to liver problems and even cancer risks. The frying pan had a label that sounded official enough. But the details were fuzzy. The compound in the nonstick layer slipped through the cracks, only flagged after research caught up.
Take triclosan, once in antibacterial soaps. For years, it carried a “safe” label. Scientists later found it stuck around in waterways and might mess with hormones in fish and people. It turns out, testing for one-off exposure gives only part of the picture. The real world brings a steady drip of low doses, day after day, sometimes for years. Accumulation and combined exposures often get less attention than they should.
Safety comes from real-world evidence, not lab theory alone. Toxicologists assess how much people are likely to encounter, how the body breaks it down, and whether any traces get stuck in organs. They watch for effects on children, pregnant women, and older adults, who may react differently than a healthy adult male volunteer in a trial.
Manufacturers sometimes lean on “generally recognized as safe” (GRAS) labels, based on industry-driven or older studies. Independent, peer-reviewed research offers confidence that doesn’t depend on a company’s bottom line. Studies funded by non-profit groups or universities help avoid rose-colored conclusions, which benefit only the producer.
So where do we go from here? Transparency helps. Brands need to list all ingredients, not just the ones required by law. Shoppers benefit from third-party seals—like the EPA’s Safer Choice or the EWG Verified marks. I rely on these when picking out cleaning supplies for my family.
Switching to proven, well-studied compounds over “new and improved” chemicals cuts risk. Anything added to a product should face independent review, and companies should have to show the science behind their safety claims. If new data points to danger, quick product recalls must follow, not slow debates.
Our everyday products keep changing. What won’t change: demand for safety, honest labeling, and credible science to back up the stuff we use at home. Everyone deserves peace of mind that today’s shortcut doesn’t become tomorrow’s regret.
People ask about the solubility of a product almost every day — in labs, at work, or even at home. Whether you’re formulating a cleaner, making pharmaceuticals, or just curious about how something dissolves in water, the answer to this question shapes how we use and store these products. I’ve found this myself trying to clean something off a countertop, reaching for the closest solvent, and wondering, "Will this actually work?" The short answer is: it really depends on the substance, and understanding the science changes your approach.
Let’s imagine you’re holding a bottle of an unfamiliar product. The label tells you nothing about its chemical structure or how it behaves in water or ethanol. Some products slip straight into water — sugar or salt vanish with a good stir. Others, like oils and many medicines, resist water and need something like ethanol or acetone to dissolve. The reason traces back to the old saying, "like dissolves like." Polar compounds, those with uneven charges, mix well with other polar substances such as water. Nonpolar ones, like many oils and fats, don’t stand a chance in water but dissolve quickly in nonpolar organic solvents.
This isn’t just a chemistry trivia fact. Understanding solubility protects workers in chemical plants and pharmacists at the counter. At a factory making adhesives, the wrong solvent means wasted batches and equipment gummed up with residue. At a pharmacy, picking a water-insoluble ingredient for a cough syrup leads to gritty, unusable medicine. One winter I tried cleaning model paint off my hands with warm water — a pointless task without a proper solvent. Many have felt that frustration after spilling nail polish or ink.
There’s real value in leaning on good sources for solubility data. The Merck Index, Sigma-Aldrich product briefs, and peer-reviewed journals pile up with hard numbers: grams per 100 mL, percent by weight, step-by-step protocols. Unfortunately, not all sources keep the facts straight. Too often, I’ve seen copy-pasted product specs that give a vague "soluble in water," ignore temperature, or miss that "soluble" doesn’t mean "will work in my process." Good practice means looking for data measured at real temperatures, usually 20 to 25°C, and taking note if the product also dissolves in alcohol, acetone, or something stronger.
Stubborn insoluble products push us to get creative. Sometimes heating helps; other times, emulsifiers bridge the gap between a product and water. Pharmaceutical companies often tweak molecules to boost solubility, aiming to deliver drugs that actually reach the bloodstream. In cleaning or coatings, switching solvents may fix a tacky finish or let pigments spread smoothly.
The bottom line: clear, honest info on solubility, checked with reliable sources, drives safer workplaces, better products, and less wasted time. Whenever I face an unknown product, grabbing the safety data sheet and checking the numbers first has saved plenty of money and frustration in my career.
Keeping any chemical safe and intact isn’t just about keeping a cap on the bottle. Some substances, especially complex dyes like Sodium 4-(4-Chloro-6-(N-Ethylanilino)-1,3,5-Triazin-2-Ylamino)-2-(1-(2-Chlorophenyl)-5-Hydroxy-3-Methyl-1H-Pyrazol-4-Ylazo)Benzenesulfonate, push us to think about what temperature, light, moisture, and other chemicals sit nearby. Let’s get real: when you neglect storage, you risk worker safety, product quality, and the bottom line. Nobody wants to find out their batch lost color strength—or worse, became hazardous—because of sloppy storage.
In my time working alongside lab techs and plant supervisors, I’ve seen plenty of well-meaning folks rely on guesswork. That approach backfires, especially with chemicals like this triazine-based dye. Sodium 4-(4-Chloro-6-(N-Ethylanilino)-1,3,5-Triazin-2-Ylamino)-2-(1-(2-Chlorophenyl)-5-Hydroxy-3-Methyl-1H-Pyrazol-4-Ylazo)Benzenesulfonate hates moisture and strong light. Dye molecules can break apart or clump together, creating unreliable results in textile production or research. Humidity sneaks into open containers, turning powders lumpy or sparking slow decomposition. Fumes from acids, bases, or oxidizers ruin nearby chemicals, sometimes setting off unwanted reactions.
Storing this dye at room temperature, away from heat sources, keeps its chemical backbone stable. Heat kicks off side reactions—sometimes visible as discoloration or even a strong, chemical smell. Refrigerated storage isn’t always necessary, but letting it bake under a sunlit window or near a radiator is just asking for trouble. Direct sun bleaches out color; ultraviolet rays snip at the dye’s structure. I’ve seen drums of high-value powder go bad sitting on a sunny loading dock, cost thousands to replace, and create headaches nobody enjoys.
A dry environment is just as important as keeping light away. Humidity in the air triggers some dyes to cake up, lose flow properties, or degrade. Air-tight, chemical-resistant containers with clear labeling help everyone keep track of what’s inside. Most labs use heavy-duty plastic or glass, but metal works in many cases—just steer clear of corroding lids. Every time a lid slips off or gets left loose, moisture and contaminants rush in. Desiccant packets inside storage cabinets soak up extra humidity, an easy trick I learned early in my career.
Storing chemicals together just to save shelf space invites disaster. Strong acids, bases, and oxidizing agents should stay away from this dye powder. Cross-contamination makes cleanup complicated and can void quality assurance. Store flammable solvents and reactive ingredients in separate cabinets. I’ve watched audits flag simple mix-ups—a bottle of dye sitting by a bleach drum means downtime, paperwork, and sometimes fines. It’s easier to keep labels bold and store each category apart.
Written guidelines or checklists help, but training makes the rules stick. Safety data sheets give reliable storage tips, and everyone working with complex dyes should know where they are. Regular inspections spot leaks, faded labels, or misplaced items before they become problems. In my experience, nothing beats a short daily check—five minutes can save a lot of hassle. A clean, organized storage room reflects pride in the workplace and sends the right signal to anyone visiting the site.
Paying attention to how you store Sodium 4-(4-Chloro-6-(N-Ethylanilino)-1,3,5-Triazin-2-Ylamino)-2-(1-(2-Chlorophenyl)-5-Hydroxy-3-Methyl-1H-Pyrazol-4-Ylazo)Benzenesulfonate protects people, products, and reputations. Every step, from labeling to lid-tightening, pays off in safety and results.
Simple products at home or work often contain ingredients many people never question. Some of these substances come from natural sources. Others rely on complicated chemical manufacturing. Years ago, I was shocked to learn that a common cleaner under my sink reacted dangerously with a perfectly ordinary bathroom product. Mixing them released chlorine gas. The hazy warning labels did little to explain the actual risk. Understanding what’s in a bottle, paint can, or snack wrapper makes a real difference.
Chemicals often don’t vanish after use. Take per- and polyfluoroalkyl substances (PFAS), used in water-resistant clothes and non-stick pans. PFAS build up over decades in soil and water. Their byproducts filter into streams, rivers, and even tap water. Research from the Environmental Working Group found PFAS in the drinking water of over 200 million Americans. Once inside the body, certain chemicals may cause issues ranging from mild irritation to higher risks of cancer or hormone disruption. For me, the idea that trace amounts of industrial waste could end up in my blood is more unsettling than the headlines about polluted landfills.
During a summer job at a hardware store, I handled bags of fertilizer and pesticides all day. I remember the sharp smell sticking to my skin and clothes. A headache crept on if I worked too close to the bags without gloves. Only later did I discover that some ingredients could seep into groundwater and threaten not just the immediate surroundings but people living miles away. In rural towns, well water sometimes tests positive for nitrates linked to run-off from farms, which can threaten infants and elderly health.
It’s easy to toss a plastic bottle into the trash and not think where it goes. Many plastics contain bisphenol A (BPA) or phthalates, seepage from plastics into food and water raises health alarms. Researchers at the National Institutes of Health found microplastics in nearly every watershed they studied. Studies suggest a connection to immune system and reproductive issues in both animals and people. Problems like air pollution aren’t always visible, either. Cleaning sprays, air fresheners, or paints can release volatile organic compounds (VOCs). These compounds cause headaches at low doses and more serious lung problems with long-term exposure.
People don’t have to become chemists to protect themselves. Small choices add up: switching to green household products, supporting brands that disclose ingredients, or simply airing out a room after cleaning can reduce exposure. Communities have made progress banning some hazardous chemicals from children’s toys and phasing out harmful pesticides. Reading reliable labels, checking scientific resources, and asking questions about ingredients makes a bigger impact than most realize.
We all share in the outcomes—healthier communities, safer work environments, and cleaner water. No one can control every risk, but informed choices and honest discussions help keep families and neighborhoods safe from hazards that might not make headlines.
| Names | |
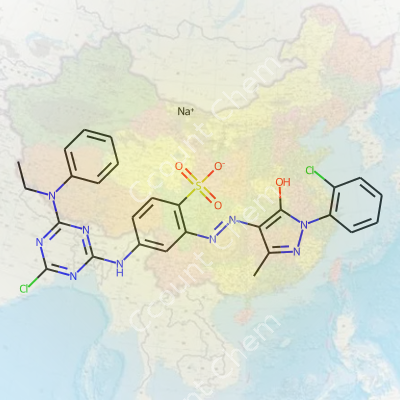
| Preferred IUPAC name | Sodium 4-[(4-chloro-6-(N-ethylanilino)-1,3,5-triazin-2-yl)amino]-2-[(E)-{1-(2-chlorophenyl)-5-hydroxy-3-methyl-1H-pyrazol-4-yl}diazenyl]benzenesulfonate |
| Other names |
C.I. Reactive Orange 16 Reactive Orange WO Reactive Orange 4GL |
| Pronunciation | /ˈsəʊdiəm fɔːr fɔːr ˈklɔːrəʊ sɪks ɛn ˈɛθɪlˌænɪliːnəʊ wʌn θri faɪv traɪˈəzɪn tuː ɪlˈæmɪnəʊ tuː wʌn tuː ˈklɔːrəʊˌfiːnɪl faɪv ˈhaɪdrɒksi θri ˈmiːθəl wʌn eɪtʃ paɪˈræzɒl fɔːr ˈjaːzəʊ benzˈsiːnsʌlˌfəʊneɪt/ |
| Identifiers | |
| CAS Number | [14426-45-2] |
| 3D model (JSmol) | `3Dmol:smi[CCNc1cc(nc(Nc2cc(S(=O)(=O)[O-])ccc2N=Nc2c(C)cnn2-c2ccccc2Cl)n1Cl)Cl]` |
| Beilstein Reference | 11112531 |
| ChEBI | CHEBI:91222 |
| ChEMBL | CHEMBL2103839 |
| ChemSpider | 24710487 |
| DrugBank | DB08715 |
| ECHA InfoCard | 01f8b568-7d7c-4cef-85dd-970a25b10636 |
| EC Number | EC 401-050-7 |
| Gmelin Reference | 104135 |
| KEGG | C22123031 |
| MeSH | Dyes, Biological |
| PubChem CID | 132444875 |
| RTECS number | GV7950000 |
| UNII | 13S4I3323S |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | DTXSID8056653 |
| Properties | |
| Chemical formula | C25H21Cl2N8NaO4S |
| Molar mass | 755.21 g/mol |
| Appearance | Red powder |
| Odor | Odorless |
| Density | Density: 1.5 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.8 |
| Basicity (pKb) | 12.56 |
| Magnetic susceptibility (χ) | -53.0e-6 cm³/mol |
| Refractive index (nD) | 1.663 |
| Dipole moment | 7.7 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 491.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | ΔfH⦵298 = -1057.3 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V04CH30 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313, P302+P352, P332+P313, P362 |
| Flash point | > 105 °C |
| Lethal dose or concentration | LD50 Oral Rat > 2000 mg/kg |
| LD50 (median dose) | 2400 mg/kg (rat, oral) |
| NIOSH | WT2769000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 120 mg/L |
| Related compounds | |
| Related compounds |
Disperse Yellow 42 Reactive Red 120 Acid Yellow 36 Direct Blue 86 C.I. Reactive Blue 19 |