Progress in biological sciences often rides on the backs of sturdy, reliable compounds. Sodium 4-(2-Hydroxyethyl)Piperazin-1-Ylethanesulphonate—better known as HEPES sodium salt—started turning up in the late 1960s, born out of a laboratory need to keep pH steady during experiments with living cells. The “Good’s buffers,” named after Norman Good who helped develop a series of zwitterionic buffering agents, revolutionized the way researchers maintained cell cultures. Before HEPES came on the scene, many labs worried over shifting pH values that killed experimental accuracy, especially for living tissues out of their comfort zone. Roughly sixty years later, people working in molecular biology, clinical diagnostics, and pharmaceutical research still turn to this buffer, trusting its gentle touch and stability under typical experimental workloads.
HEPES sodium salt wears many hats in the laboratory. The compound acts as a strong buffering agent. Most folks buy it in white crystalline powder form, which dissolves easily in water and creates clear, odorless solutions. When I first started out in cell culture, this was a go-to on the bench because it kept my cells comfortable, especially under the heat of a 37°C incubator or in the presence of CO2. It hardly interacts with metal ions or common reagents, which is huge for sensitive molecular assays or enzyme-driven tests. Many culture media and diagnostic solutions list HEPES sodium as an ingredient, not because it's flashy, but because it shows up day after day—unchanged and reliable.
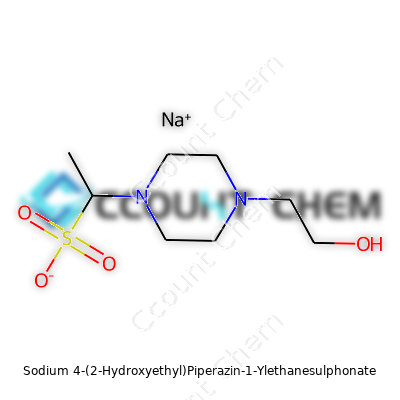
The compound’s molecular formula is C8H17N2NaO4S and a molar mass of roughly 260 g/mol. Solid HEPES sodium salt sits as a fine, free-flowing white powder that draws in little moisture from the air, making storage less of a headache. It dissolves quickly in water, turning clear without cloudiness. The real value, though, lies in its pKa close to 7.55 at 25°C. This number puts its optimal buffering range smack in the middle of what most mammalian cells crave: just above neutral pH. That means whether folks are running a flow cytometry experiment or maintaining a row of organoids, the buffer does its work without adding background “noise.”
Suppliers who know their customers' needs provide detailed labeling—batch number, purity (usually above 99%), and moisture content. Experienced users always look for heavy metals and endotoxin testing, especially in biopharma and clinical labs. Some regulations require USP or EP-grade materials for critical medical or diagnostic use. I once learned the hard way that even a small impurity can throw off expensive experiments. That’s why I always check that labels call out the sodium salt form and not just generically “HEPES,” as the free acid and sodium salt dissolve and behave differently.
Labs and manufacturers create HEPES sodium salt using straightforward organic synthesis. First, they make HEPES base through the reaction of ethylene oxide with piperazine—two chemicals found in bigger chemical plants. Then, they neutralize it with sodium hydroxide to switch out the acidic hydrogen for a sodium atom. Careful dialysis or recrystallization helps purify the final product. At each step, technicians check for formation of by-products which could interfere with sensitive biological systems. Over the years, automation and scaled-up processes made these reactions faster and more consistent, slashing costs for end users. This progress helped expand buffer use to larger and more complicated applications, like manufacturing vaccines or cell therapies.
Though stable under neutral conditions, HEPES sodium salt reacts to extreme heat and acidic or basic pH. It rarely takes part in unwanted side reactions in water—one of its main selling points compared to older buffers. Still, under high-energy conditions like autoclaving, those piperazine rings can slowly break down, producing small amounts of hydrogen peroxide. Most cell culture folks I know avoid autoclaving it for that reason, preferring either to filter-sterilize solutions or prepare them fresh. Chemists sometimes modify the ethyl or hydroxyethyl side chains to tune buffering range or solubility, creating analogs for specialty needs. Even as HEPES, the sodium salt itself offers a relatively clean slate, rarely clouded by reactions with enzymes or cofactors.
Navigating supplier catalogs means learning a handful of names. Some call it HEPES sodium, others write out the whole chemical mouthful, but it also goes by 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid sodium salt. Popular brands label it just “HEPES-Na,” and research papers sometimes say “HEPES buffer sodium salt.” Checking the packaging for CAS Number 75277-39-3 kept me from ordering the wrong variant. Across the major chemical distributors and medical supply services, these names signal nearly identical products, though quality grades and certifications may differ.
Modern users pay close attention to hazard warnings. HEPES sodium salt by itself ranks as low risk—no fumes, low toxicity, and non-flammable. During routine use, basic protective gear like gloves and lab coats more than suffice. Any inhalation or ingestion risks are low compared to corrosive or volatile chemicals, but it is still wise to avoid exposure. I once dropped a batch on the floor and got by with a shop vac and a patient janitor. Manufacturers stick to ISO and GMP standards in production, especially where the buffer ends up in medical or diagnostic tools. Material Safety Data Sheets back up claims about safe storage, handling, and what to do if someone splashes the buffer into their eyes.
This buffer now serves in hundreds of routine protocols. Clinical blood analyzers, tissue and cell culture systems, protein purification kits, and vaccine production lines all rely on HEPES sodium salt. Some researchers even use it as a supporting buffer in electrophysiology and imaging because of its minimal autofluorescence and steady pH. Larger-scale production of gene therapies and cell-based medicines benefit from its consistent performance and low toxicity to living cells. Environmental testing, veterinary diagnostics, and food safety labs use HEPES sodium salt to control pH where accuracy beats speed. My own work involved using it in flow cytometry staining buffers, where small drift in pH could ruin cell viability or mask valuable surface markers. Having a buffer that just works kept experiments on time and budgets intact.
Ongoing innovation pushes buffer design forward. Some teams in pharmaceutical development tweak HEPES sodium salt or blend it with other buffering agents to match niche environments—cold storage, rapid pH shifts, or multi-enzyme systems. Analytical chemists continue probing the long-term stability of HEPES sodium in high-throughput formats. Advances in biomanufacturing put extra pressure on every component in the workflow—if a single additive lacks reliability, the cost and time to troubleshoot can send projects sideways. That steady reliability, proven across thousands of publications, draws new researchers into trying HEPES sodium salt for the first time instead of experimenting with less-studied alternatives.
Through decades of lab use, researchers cataloged toxicity data for HEPES sodium salt. Animal studies suggest the compound produces low acute toxicity, rarely triggering significant side effects at concentrations used in tissue or cell culture. Long-term exposure studies are less common, but the compound does not bioaccumulate or break down into persistent toxins. Regulators still recommend avoiding direct injection into living systems unless validated under strict controls—an approach mirrored by industry standards for similar biological buffers. Any stories you hear about cell line sensitivities usually trace back to overuse at concentrations above 50 mM or improper sterilization.
Demand for strong buffers like HEPES sodium salt shows no sign of shrinking. Growing use in cell and gene therapy, rapid diagnostic platforms, and next-generation biologics places a premium on chemical tools that just work. Thoughtful innovation could bring lower-cost, higher-purity blends as supply chains modernize. Some sustainability projects target greener synthesis with fewer byproducts and tighter recycling loops. Academic studies may one day reveal even more precise pH controls or allow automation in process development. For now, HEPES sodium salt continues to show up on benches, in protocols, and even in global public health projects, proving that an old standby with a reliable record still finds new jobs to do.
Mention sodium 4-(2-hydroxyethyl)piperazin-1-ylethanesulphonate among biologists and chemists, and most will just call it HEPES. The long scientific label hides a simple truth: this is one of the more steady buffers hanging out in lab benches worldwide. Buffers matter because living cells can’t stand sudden swings in pH, and this chemical’s job in labs boils down to keeping things steady. Swap in HEPES, and cell cultures rarely complain.
Anyone with experience running cell cultures knows that not every buffer gets along with life, especially under harsh fluorescents and hot incubators. Ordinary buffers sometimes lose control of pH if exposed to the air too long. CO₂ levels mess with some of them, and you’re left scratching your head over weird results. HEPES doesn’t stumble here. It shrugs off changes in carbon dioxide and stays solid at the pH sweet spot—usually around 7.2 to 7.6. Some published research shows it even extends the window for keeping cells alive, which means fewer ruined experiments and happier grad students.
Tissue culture rooms use HEPES in their media, but you’ll run into it in protein research and molecular biology too. Run a Western blot or purify proteins, and this buffer can streamline the hassle. I’ve watched researchers go HEPES-heavy when they needed crystal-clear results, especially where even a small drift in acidity could throw off the whole run.
Not every buffer juggles all these variables. HEPES doesn’t absorb much light at the wavelengths scientists use to measure protein or DNA. That allows more freedom to track reactions inside clear liquid tubes without interference. Imagine standing over a spectrophotometer and knowing your readings aren’t getting skewed by the buffer. That builds trust in your results, making grant writing and paper publishing less stressful.
Some day-to-day examples stick in my memory. We once tried running electrophysiology experiments with other buffers and hit patchy signals every time the air conditioning cycled on. Switching to HEPES, the system stopped spiking. Cells seemed happier, the baseline steadied. That’s the kind of real-world payoff scientists crave.
For all its reliability, HEPES can raise some eyebrows. It isn’t perfect, especially if left in sunlight for too long since it might break down and form a few toxic byproducts. There’s also the question of cost. Lab budgets feel the pinch, and HEPES costs real money. Some biotech firms consider alternatives or mix low and high-cost buffers together just to save a few bucks. Lab technicians and researchers have a responsibility to dispose of it safely because wastewater treatment plants can’t always handle these complex molecules.
Labs will always chase stable results, so HEPES isn’t going anywhere. Smarter use, paired with ongoing research into safer, cheaper buffers, could ease environmental headaches and keep everyone’s research rolling. If the science community starts prioritizing both cell survival and what happens after the experiment ends, everyone benefits—from the cells all the way up to public health.
Storing products in the right environment directly protects their quality and safety. Temperature, humidity, exposure to light—these factors shape shelf life much more than most labels suggest. A mistake in storage can reduce effectiveness, change color, spoil taste, or encourage bacteria and mold. In my years of working with suppliers and checking shipments, I’ve seen everything from wilted fresh produce to crystallized medicines just because the truck or warehouse ran a few degrees too warm. Product safety starts after production, not before it lands in a customer’s hands.
Heat speeds up chemical break-down, so drugs, supplements, and cosmetics often call for "store in a cool, dry place." Cool doesn’t mean cold; for most products, it points to room temperature—between 15 to 25 degrees Celsius (59 to 77 degrees Fahrenheit). That’s what prevents pills from sticking together or chocolates from blooming white. Humidity brings its own issues: it clumps powders, warps packaging, and encourages mold or microbes. For this reason, a dry space with low humidity—usually under 60%—guards against spoilage. Pharmacy fridges need regular checks because even a short power cut will let sensitive shots and serums slip out of a safe temperature zone.
Sunlight and even harsh indoor lights can break down vitamins, cause oils to turn rancid, or bleach colors. Think of how perfumes fade if left on a sunny windowsill. Opaque packaging and closed cabinets go a long way. I remember visiting a family friend who always topped off his olive oil in a clear glass bottle on the counter. After six months, the oil tasted flat and bitter from light damage. Airtight containers stop exposure to oxygen, which can dry out, oxidize, or spoil many goods. For foods, a well-closed package maintains crispness and flavor. For longer-term storage, vacuum-sealing stretches shelf life even more.
Dust, pests, and stray odors turn any storage space into a hazard for unprotected products. Keeping storage shelves, drawers, and refrigerators spotless matters just as much as maintaining the right temperature. Simple habits like washing hands, using clean scoops, and rotating stock so older items are used first, can prevent contamination. Avoiding strong-smelling chemicals or cleaners nearby protects the taste and scent of food or fragrance products. I once kept apples in the fridge next to a bag of onions, and the fruit absorbed the strong smell within days.
Manufacturers usually include clear guidelines, sometimes even icons, on labels. People sometimes miss this small print, which leads to storage mistakes. I’ve found it helpful to label shelves or drawers in the kitchen and medicine cabinet with simple signs: “dry goods only," "refrigerate below 8°C," or "keep away from sunlight.” If products have specific needs—such as insulin vials or freshly mixed baby formula—reading the producer’s advice and following it precisely creates better results and much less waste.
Small climate monitors and hygrometers, available online and in hardware stores, give quick feedback on a cupboard or warehouse’s environment. Investing in airtight jars, dark glass bottles, or even basic food-grade bins costs little compared to replacing spoiled items. In businesses, regular training sessions and checklists keep staff alert to seasonal changes in storage conditions. On the home front, building a routine—checking fridge temperature, keeping lids tightly closed, cleaning out expired items—brings peace of mind and a longer life for your goods.
Working with cell cultures gets tricky fast, especially if you plan to introduce a new compound into the mix. In my own work at the bench, I’ve watched how a minor ingredient can throw off an entire week’s worth of progress. Beyond just “keeping cells alive,” the question circles back to whether the compound actually lets cells grow and function in ways you’d expect. That’s more than just basic compatibility. It means looking at toxicity, stability, predictability, and even downstream effects.
Plenty of folks assume a compound is fine for lab use if it's been through the filter of a chemical supplier or shows up in published recipes. Yet, I’ve seen how a small difference—like the source of a buffer or the batch number of a supplement—can set up unwanted surprises. For example, serum that’s handled differently between shipments can mean the difference between healthy, spreading cells and a culture full of sad, detached blobs. Apply that thinking to compounds, and the stakes rise quickly.
The first question always comes down to toxicity. Chemical lists and supplier catalog entries usually won’t tell you how a certain compound behaves inside a live, dividing cell. Lab experience tells a clearer story: even small impurities or a change in pH can quickly damage fragile lines, especially primary isolates. According to a survey published by Nature in 2018, over 60% of cell culture contamination events result from unnoticed ingredients or unvetted additives. Relying on a trusted, cell-culture tested certificate gives a stronger base, yet lab verification still matters.
Stability is another big deal in the real world. Some chemicals look fine at the time you make a stock solution. After a cycle of freeze-thaw or just sitting in the light for a day, breakdown products can start slamming your cultures. I learned early on that even something as simple as glutamine needs a fresh mix, otherwise ammonia buildup starts to kill cells faster than you can troubleshoot. Bleaching a plate of HeLa cells because of old media is no fun, and it’s a mistake you only need to make once.
Most people tend to forget that different cell lines react in wildly different ways to the same substance. A cancer line might shrug off a chemical that would wipe out a delicate neuron or iPSC. Referencing published work on your exact line and application can save a lot of frustration. If peer-reviewed papers haven’t used the compound in a similar setting, it’s wise to proceed with extra caution and keep tight controls.
Beyond just not killing the cells, compatibility lines up with the research goal. For drug discovery or metabolism research, even subtle effects on pathways or off-target actions can sink the whole project. Teams at pharmaceutical companies set up multi-step screens: cytotoxicity, then metabolic effects, then phenotype tracking, all to catch problems early. The average academic lab rarely runs all those checks. That gap can lead to headaches down the line.
If there’s doubt about a compound, start with a low dose and a small-scale test. Document everything—media batch, cell lot, passage number. Use trusted positive and negative controls. If anything seems off, ask suppliers for validation reports or chemical purity data. Peer-reviewed journals and research-focused suppliers usually offer cleaner, better-tracked products, making life easier for anyone trying to stick to E-E-A-T standards. Above all, keep communication open with colleagues and collaborators. A story about one lab’s experience with a bad batch often saves another from repeating mistakes.
Anyone who’s spent time in a wet lab recognizes the long-winded name, Sodium 4-(2-Hydroxyethyl)Piperazin-1-Ylethanesulphonate. Most lab folks call it HEPES sodium salt. Its formula—C8H17N2NaO4S—hides a lot of chemistry. What catches my attention isn’t just the alphabet soup, but the molecular weight: 260.29 grams per mole. That number, tucked away in manufacturer catalogs and chemical safety sheets, shapes every pipette, flask, and buffer bottle that ever sees HEPES inside.
A lot of buffer recipes might seem to revolve around this detail, but the real value goes deeper. Molecular weight controls how scientists prep solutions. I remember in grad school, frustration rising as buffers drifted out of range because someone swapped out free acid for sodium salt, or mixed up the mass calculations. A solution isn’t just “some HEPES and water”—every milligram matters. The difference between 238.3 for the free acid and 260.29 for the sodium salt can make or break an experiment.
Accuracy often defines true reproducibility in research. An error in calculating how much HEPES to weigh out might not flood your gels or melt your cells, but it does easily tip a pH by several tenths. Across hundreds of experiments or in the scaled-up batches at biotech plants, this small discrepancy can skew entire sets of data. At the bench, most researchers use electronic balances precise to a fraction of a milligram. These balances rely on proper input. Get the molecular weight wrong, and the solution will miss the intended target, no matter how careful the weighing.
USP and EP monographs echo this same message: details matter. Reliable research, drug development, and diagnostics all trace back to careful chemical preparation. I’ve worked on collaborative projects where labs across the world depended on sending identical reagents. Each weighed out their buffer components, trusting everyone else used the same value for molecular weight. If only one lab miscoded it—mistaking HEPES free acid for sodium salt—the outcomes went haywire. Science works because numbers like 260.29 mean the same thing everywhere.
Misreading the label or using an outdated data source still fouls up labs. There’s a growing push for digital lab notebooks that automate this part of the process. Instead of relying on memory or outdated handbooks, a quick lookup runs through a trusted chemical database. Not everyone has such setups, though. Old-school labs still keep Merck or Sigma-Aldrich catalogs within arm’s reach, flipping to the physical property charts to double check a molecular weight—an unglamorous but lifesaving habit.
Training matters too. Early in my research days, a PI hammered into us the wisdom of double-checking every number. Cross-referencing molecular weight doesn’t just avoid waste; it preserves trust in the results, for publication and for colleagues trying to replicate what we’ve done.
Whether helping med students mix the perfect buffer or prepping solutions meant for sensitive cell culture, a five-minute check on molecular weight avoids wasted work and confusion down the line. That 260.29, scribbled on the label, might seem a minor chemistry detail. In reality, it forms the backbone of everyday good science—one batch, one buffer, one experiment at a time.
Taking a closer look at the safety of a product can save a lot of trouble down the road. Years ago, a neighbor bought a cheap off-brand cleaning spray at a discount store. Nobody looked twice at the label—until her child landed in the ER after a rash developed. Turns out, it had a chemical banned in the EU. That story hits close every time I check the back of a new bottle, whether it’s floor cleaner or face wash. Hazardous products don’t sit on shelves just in the movies.
Hazards might include toxic fumes, chemical burns, allergic reactions, or worse, slow-acting threats nobody spots until it’s a problem. The U.S. Consumer Product Safety Commission flagged over 400 products for recalls just last year, and not all of them were fireworks or flammable batteries. Items like children’s clothing, kitchen tools, pet food—real ordinary stuff—made that list. It’s easy to fall into the trap of thinking big-name brands guarantee safety, but recalls hit them too. The market’s size means mistakes slip through, and sometimes marketing glosses over real risks.
A product label can tip you off. Ingredients with long, hard-to-pronounce names don’t always spell trouble, but names like “formaldehyde” or “phthalates” are worth a pause. According to the National Institutes of Health, exposure to formaldehyde links to headaches, asthma attacks, and cancer if there’s enough over time. Personal care items may hide such ingredients under umbrella terms like “fragrance”—one word, a lot of questions.
I keep a habit these days: ten seconds with a search bar before buying anything new. The EPA offers a Safer Choice database that lists products cleared for home use; their site is user-friendly and skips confusing chemical jargon. Consumer Reports and nonprofit watchdogs also publish easy-to-read guides on everyday products— from cookware to kids’ toys. The information isn’t just for the super-cautious; it uncovers stories of recalled items before the news even breaks. Crowdsourcing reviews and safety complaints adds a real-world touch missing from polished advertisements.
Companies respond when people start demanding to know what goes into a product. People opting for items certified by third parties—like the EcoLogo, the Green Seal, or the UL mark—send a message to brands. These certifications require independent testing with proven standards in place. Ending up with a safer kitchen tool or a gentle shampoo doesn’t need a chemistry degree—just a bit of homework and keeping your eyes open.
On the policy side, rules get tougher year by year, but loopholes remain. Pushing for full ingredient disclosure and sharper labeling requirements is long overdue. Smarter regulation and more public transparency in labeling would allow everyone to make better decisions at the point of sale. Until then, checking safety ratings and side effects before sharing a product with kids, pets, or anyone with health conditions looks less like paranoia and more like common sense.
Staying informed counts as self-care. A quick label check could spare a world of hassle down the line, and that’s something everyone can get behind.

| Names | |
| Preferred IUPAC name | Sodium 2-[2-hydroxyethyl(piperazin-1-yl)]ethane-1-sulfonate |
| Other names |
HEPES 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid |
| Pronunciation | /ˈsəʊdiəm ˈfɔːr ˈtuː haɪˈdrɒksiˌɛθɪl pɪpəˈreɪzɪn wʌn aɪl ˈɛθeɪnˌsʌlˌfəˌneɪt/ |
| Identifiers | |
| CAS Number | 7365-45-9 |
| Beilstein Reference | 3587262 |
| ChEBI | CHEBI:9129 |
| ChEMBL | CHEMBL1350 |
| ChemSpider | 12036 |
| DrugBank | DB02145 |
| ECHA InfoCard | 03-2110-0068 |
| EC Number | EC 220-876-7 |
| Gmelin Reference | 84889 |
| KEGG | C05633 |
| MeSH | D017379 |
| PubChem CID | 23665474 |
| RTECS number | TC6300000 |
| UNII | YUX0L6K1Y5 |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | Q27116243 |
| Properties | |
| Chemical formula | C6H14N2O4S |
| Molar mass | 292.38 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.11 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.5 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 7.5 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -5.7×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.497 |
| Dipole moment | 6.44 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 299.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2273.7 kJ/mol |
| Pharmacology | |
| ATC code | B05XA15 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | Precautionary statements: P264, P280, P305+P351+P338, P337+P313 |
| Flash point | > 196.7 °C |
| Lethal dose or concentration | LD50 Oral - Rat - > 10,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat >9,000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20-25°C |
| Related compounds | |
| Related compounds |
HEPES MES MOPS PIPES TES Tris ACES BES CHES EPPS |