The story of sodium 3-mercaptopropanesulphonate tracks the path of chemical innovation in the electroplating industry. Chemists in the mid-twentieth century looked for agents that could smooth and refine nickel and copper deposits, aiming to leave behind the cloudy finishes that plagued early products. Electrolyte baths in those days often threw up unpredictable results, and the hunt for more reliable plating agents led researchers to study organic sulfonic acids with active thiol groups. After trials and a few false starts, laboratories realized that this specific compound brought the right combination of water solubility, stability, and the mysterious tendency to influence metal crystal shapes. Industry rapidly picked up on its benefits. Over the decades, manufacturers tweaked formulations, but the compound still sits on many plating shop shelves today. That journey tells us a lot: necessity pushes discovery, but persistence weeds out what doesn't work in real life.
Sodium 3-mercaptopropanesulphonate carries a unique dual-functional structure. One end holds a sulfonate group, sticker than superglue in water, the other bristles with a sulfur atom primed for chemical action. Chemists rely on this compound as a bath additive, mostly in the electroplating of metals like copper and tin. Some folks call it SPS for short. You’ll also see it listed under various product names, depending on the region and the supplier, including mercaptosulfonate sodium, which basically reads as a direct translation of its chemical bones. The business behind this compound is straightforward: consistent supply and reliable chemical purity mean plating companies get predictable outcomes. It’s not a boutique molecule but a workhorse that plenty of people rarely talk about, unless something goes wrong in the process.
As a white, free-flowing crystalline powder, sodium 3-mercaptopropanesulphonate dissolves easily in water, making it fit right into aqueous plating baths. It brings a faint, sulfuric sense to the nose, something anyone in a plating workshop comes to recognize quickly. With a molecular weight hovering around 178 grams per mole, it doesn’t offer up much in terms of visual drama but gets the job done. Its melting point sits comfortably above typical room temperatures, meaning it won’t break down under normal storage or use. The chemical also remains remarkably stable in standard skin-contact or environmental conditions, resisting breakdown unless exposed to intense heat or strong acidic/alkaline environments. The mercapto (-SH) group is more than a decorative flourish: it grabs hard onto metal ions, affecting how tiny copper or tin grains knit themselves together on cathodes.
Manufacturers don’t leave labeling to chance. Product sheets give purity figures, usually north of 98%, and spell out trace impurity content. Moisture control is critical; too much water and the additive clumps or reacts, too little and it cakes. Bags and drums come stamped with batch numbers and expiration dates, tracked back to each production run. Safety data sheets ride along with every order, outlining not just handling tips but legal requirements for shipping and use. Customers look for lot consistency above all. Regulatory compliance plays a role, especially for export, since countries expect support documents on heavy metal content, allergen potential, and compliance with worker safety rules like OSHA or REACH. Factories regularly check incoming shipments and keep an eagle eye for anything that deviates from standard.
The most common route to synthesizing sodium 3-mercaptopropanesulphonate starts with 3-chloropropanesulfonic acid sodium salt. In a classic substitution reaction, a controlled addition of sodium hydrosulfide replaces the chlorine atom with a sulfur-hydrogen group, releasing sodium chloride as a byproduct. Reaction vessels need good heat control because too much temperature spikes yield poor product and dangerous byproducts. Chemists monitor pH and stir the mixture to keep things even. The process doesn’t forgive sloppiness. After reaction, filtration removes salt, then the product gets dried, milled, and monitored for purity. Scale manufacturers separate themselves from the pack by keeping steps clean, efficient, and reproducible, since impurities can poison a whole plating bath and cost companies dearly in lost product.
This compound proves its worth in the way it reacts. In electroplating baths, the sulfur group lines up with metal ions, reducing internal stress in deposited metals. It can undergo oxidation, with the thiol group converting to disulfide under specific conditions, shifting the performance in plating. Chemists sometimes modify this basic molecule, tacking on longer chains or varying the sulfonate group to tune how active it grows in bath environments. As a compound, it doesn’t just float around—it directs the show, steering how metal grains assemble themselves, which influences ductility, conductivity, and final product appearance. Ongoing research into its analogs and reaction products leads to newer, more selective agents every few years.
Sodium 3-mercaptopropanesulphonate has been juggled through a maze of chemical names. You might spot it listed as sodium 3-sulfanylpropane-1-sulfonate, 3-sulfopropyl mercaptan sodium salt, or even simply SPS in technical jargon. Region and manufacturer play a part in how it shows up on packaging, which sometimes can trip up new buyers looking for the right grade. Every main supplier leans into their own branding, sometimes making it a chore to match specs between catalogs. What it means in practice is simple: reading the data sheet, not just the name, makes the difference between buying what you need and ending up with the wrong stuff entirely.
Training young workers on safety with sodium 3-mercaptopropanesulphonate keeps accidents down. Dust, like with any powder, can irritate eyes or noses if left unchecked. Wearing gloves and goggles isn’t just bureaucratic—it prevents small but painful accidents. Handling and storage in dry, well-ventilated places, away from incompatible materials like strong oxidants, keeps risk to a minimum. Spills need prompt cleaning, with plain water sufficing in most cases. Disposal, under modern regulations, means collected spills or expired product shouldn’t end up in regular trash. Companies follow local hazardous material policies, tracking outgoing waste. Regular safety audits keep everyone sharp. Looking out for coworkers means double-checking labels, keeping stations clean, and minding that a small mistake with chemicals scales up quickly in a production setting.
Walk into a plating facility and you spot sodium 3-mercaptopropanesulphonate everywhere copper electroplating happens. Printed circuit boards rely on this material to ensure the copper layer stays smooth and uniform, with trace levels heavily influencing the product’s electrical reliability. Decorative finishes on metals like faucets or hardware reach their shine partly due to this chemical, driving down internal stress and grain size. Modern manufacturing settings know that even minor tweaks in concentration impact whether plated parts pass or fail rigorous quality checks, especially when filling fine features like blind holes. Lab research keeps pushing boundaries, using this compound in developing brighter, denser coatings and figuring out how to plate new alloys for the electronics and automotive sectors.
Research labs, both academic and private, keep poking at new additives and derivatives. A common approach involves mapping how changing sodium 3-mercaptopropanesulphonate concentrations tweak metal deposit profiles. My own work in a university setting showed that even slight shifts in additive levels changed grain boundaries, impacting everything from corrosion resistance to how easily a part could be soldered. Efforts in green chemistry investigate alternative production routes aiming to reduce waste and hazardous byproducts while still supplying the large industrial quantities the electronics sector expects. Some researchers look into computer models, using molecular dynamics simulations to predict how this compound’s molecules interact with growing metal films. The next round of innovation could see more targeted derivatives taking over, offering sharper control or faster deposition rates.
Reviews of sodium 3-mercaptopropanesulphonate toxicity suggest it carries a relatively low risk in standard operating conditions, though any thiol-based compound can cause issues if mishandled. Studies on its breakdown products, especially over long lifespans in high-temperature or acidic environments, raise occasional eyebrows, prompting periodic re-examination of exposure limits. Workers seldom develop direct reactions, but consistent training and industrial hygiene practices cut down on skin and respiratory complaints. Animal studies pushed to high doses show minor irritation but not the kind of acute toxicity linked to more notorious plating agents of the past. What matters in daily shop-floor life is ventilation, prompt cleanup, and a culture of reporting odd symptoms early. Occupational health researchers continue to monitor for rare sensitization or allergic responses, but such risks stay firmly in check given current practices.
Technology doesn’t stand still, and sodium 3-mercaptopropanesulphonate finds itself at a crossroads. Sustainability pressures push suppliers to explore routes with fewer emissions. Industrial robotics and tighter process controls demand even purer, more predictable additives. Interest grows in bio-derived or biodegradable replacements that keep plating quality high without lingering in the environment. Next-generation electronics, with intricate circuits and demanding miniaturization, drive ongoing demand for ultra-smooth, reliable copper layers. Ongoing innovation in the sector looks to couple well-established performance with an eco-friendlier lifecycle. While this compound has earned its place from years of solid service, the push for improved workplace safety, waste reduction, and product quality will likely lead to judicious refinement, if not outright reinvention, of essential additives like SPS in the years ahead.
Sodium 3-mercaptopropanesulphonate never gets headlines, but it quietly shapes some of the things we touch, drive, and depend on every day. I remember first coming across this compound back in a university chemistry lab as a part of an electroplating experiment. It stood out—not just for its long name or that distinct sulfur smell—but for how quickly a seemingly minor additive could fine-tune a metal coating. Most folks never meet sodium 3-mercaptopropanesulphonate by name, though nearly everyone uses things produced with its help.
Let’s talk about the auto parts and electronics that fill our lives. Copper plating runs underneath most circuit boards, while car bumpers often shine because of nickel coatings made in giant industrial tanks. Sodium 3-mercaptopropanesulphonate acts as a brightener and grain refiner in these processes. What does that mean in plain English? It helps copper and nickel form smooth, fine-grained layers, not rough or patchy ones. Smaller grains make tougher, more reliable coatings. Smoother surfaces result in less electrical resistance, sharper finishes, and longer life. These aren’t just perks—they keep down costs on maintenance and extend product life, saving tons of money for manufacturers and families alike.
Engineers often face a dilemma—choose between pricier additives or settle for weaker final products. Sodium 3-mercaptopropanesulphonate solves that by offering reliable results without a huge bill. For nickel and copper electroplating, it means fewer defects, better adhesion, and a finish that holds up to heat or humidity. Facts back this up—plating baths with this additive slash rejects and improve metal hardness compared to those without.
And it doesn’t stop at metals. Some newer research pushes this compound into battery technology, where its sulfur and sulfonate groups can control metal deposition on battery anodes. This could prevent battery failures, fires, and help new kinds of batteries last longer and charge faster. That’s a technical leap, built on decades of careful lab and industry work.
No one claims sodium 3-mercaptopropanesulphonate solves every problem in metal finishing. It can throw off the chemistry if overused and must be handled with care, both for safety and for environmental reasons. Waste from plating shops still needs proper treatment, since the breakdown products can harm aquatic life. From my contacts in industry, I hear a call for better protocols—real-time sensors, smarter recycling systems, and stricter checks. There’s a need for companies to not only meet local rules but also put money toward cleaner methods and safer workplaces.
In my experience, chemicals like sodium 3-mercaptopropanesulphonate thrive because they meet real, practical demands. Society’s drive for faster gadgets, cleaner cars, and tougher coins keeps the demand strong. Smarter use, responsible handling, and ongoing research can ensure we get all the benefits without the baggage. For companies, that means tracking performance data, investing in employee training, and keeping tabs on emerging science. For the rest of us, it means the phones in our pockets, the vehicles in our driveways, and the tech of tomorrow all work a little better—and last a little longer—thanks to an unassuming molecule doing its job right.
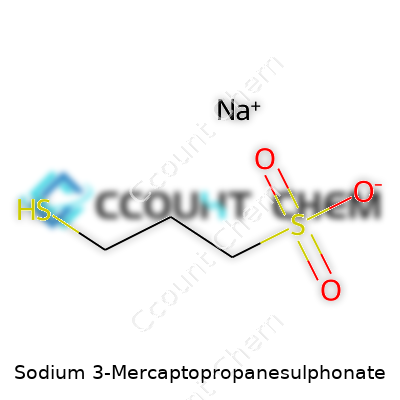
Sodium 3-mercaptopropanesulphonate has a molecular formula of C3H7NaO3S2. Its molar mass comes in at around 178.20 grams per mole. This sounds highly specific, but recognizing those numbers gives you some insight into how this compound behaves. The formula reflects three carbon atoms, a touch of sulfur, a sodium ion, and a sulfonic acid group balanced out by a thiol. That combination packs a punch, particularly in chemical processes that need reactivity and solubility all rolled together.
You’ll find sodium 3-mercaptopropanesulphonate showing up most often in the electroplating world. Just the other day I caught a conversation between two technicians at a local plating shop. They swore by its role as a brightener and grain refiner for copper and nickel plating baths. What the numbers don’t show is how this compound improves adhesion and overall plating quality without tossing health or environmental safety out the window. Instead of relying on more toxic agents, they turn to something that breaks down cleanly and is easy to rinse away.
From a health and safety standpoint, there’s peace of mind using sodium 3-mercaptopropanesulphonate. Sulfonate groups make it more water-soluble, and the sodium content helps it dissolve in the bath before interacting with the metal surface. You don’t need harsh organic solvents or nasty fumes. That’s huge for anyone working eight-hour shifts around these chemicals.
The reach doesn’t stop with metal finishing. Water treatment specialists often add this compound to trap and remove heavy metals. The thiol group snags metal ions, locking them up and making it easier to filter contaminants out of drinking supplies. With contamination creeping up in groundwater near old industrial sites, something that takes harmful metals out of the equation has a practical and direct value.
In my own experience dabbling with metalworking chemicals, easy-to-handle compounds like sodium 3-mercaptopropanesulphonate mean fewer headaches. Storage is safer, accidental spills are less dramatic, and waste disposal becomes less of a regulatory nightmare. Stakeholders in any field appreciate saving on hazardous waste fees while staying compliant with environmental rules.
This compound tells a story about where chemical manufacturing is heading. More companies want low-toxicity, high-performance additives, and chemicals like this one fit that demand. The sodium salt form controls volatility and odor, while the combined thiol and sulfonate groups nail performance needs across industries.
For anyone working in chemistry, environmental science, or industrial settings, it’s worth considering how much a well-designed formula and manageable molecular weight can simplify day-to-day operations. This reduces risks and supports a shift toward improved workplace safety. Adoption picks up when a chemical’s benefits go hand in hand with responsible manufacturing and use.
Regulators look closely at chemical footprints. Sodium 3-mercaptopropanesulphonate has found favor because its breakdown path leads to manageable components, and its environmental persistence stays on the low end. In my experience, that’s the sort of quality that helps a product survive over the long haul—especially as watchdogs push for greener alternatives and stricter reporting requirements.
Understanding the numbers behind this compound isn’t just for lab reports. It influences safety protocols, environmental practices, and even business decisions. Choosing the right molecular friends makes all the difference in clean industry, successful research, and responsible stewardship.
In the world of chemicals, Sodium 3-Mercaptopropanesulphonate feels a bit like an unsung workhorse. Plenty of industries, especially those in metal finishing and electroplating lines, treat it as a go-to additive. The stuff is valuable—not just in cost, but in the reliability it brings. Sloppy handling can turn it from a helpful tool to an unwelcome headache. Here’s why smart storage matters.
Several years working with specialty chemicals taught me: a clean shelf or a sealed drum isn’t just housekeeping. It’s about preventing expensive mistakes. Sodium 3-Mercaptopropanesulphonate holds some sulfur-based charm, but it’s not forgiving. Exposure to moisture, heat, or even sunlight can spoil its chemical profile, leading to stinky off-gassing or crystal clumping. Ignore those details and equipment fouling or unpredictable results start to surface.
Stable temperatures bring out the best in this chemical. A room with temperatures under 30°C works well. Too much heat, and you get unnecessary decomposition, which means the substance loses its intended punch. Damp areas encourage caking or degradation. That’s a recipe for performance and safety issues. Air conditioners or dehumidifiers have made a difference in many of the laboratories and warehouses I’ve visited. Small investments up front sidestepped big repair or disposal bills later.
Wide-mouthed containers seem tempting for quick scooping, but tight-sealed, quality plastic or lined metal drums prevent contamination. Flimsy bags invite humidity and accidental spillage—something I’ve seen lead to both wasted product and awkward cleanups. Clear labeling, with date of purchase and batch numbers, streamlines tracking and keeps rotation smooth.
No chemical store is immune to clutter. Sunlight can trigger unwanted reactions in sulfur compounds. Even fluorescent lights in storage rooms do their share of harm over the months. Shelving away from windows and choosing opaque containers can cut this risk. Children, food, or flammable items shouldn’t share shelf space. Even trace mix-ups can spark unpredictable reactions.
Don’t treat it like bulk salt. Even though it isn’t the most dangerous substance in the shed, it can irritate skin or eyes. I keep gloves and splash goggles nearby in my chemical area. Spills become less of a drama with prepared gear within reach. Clean up isn’t only about mopping; it’s about keeping the storage habitually tidy so powder and residue don’t build up on shelves and floors.
The best-run facilities check inventory on schedule and avoid over-ordering. Old, expired product breeds uncertainty. Disposal is not cheap, nor is it straightforward. Connecting with local chemical disposal services early avoids fines, pollution, and strained neighborly relations.
Industry and everyday safety interlock here. Solid storage habits, simple as they seem, shield workers and the bottom line. Storing Sodium 3-Mercaptopropanesulphonate thoughtfully means less downtime, fewer surprises, and a smoother production flow. That’s what gives peace of mind through every shift.
Stories about chemical safety often sound dry, but they matter a lot, especially for folks working in labs, manufacturing plants, or even college classrooms. Sodium 3-Mercaptopropanesulphonate—sometimes just called MPS—shows up in electroplating shops and some research labs. Most of the world never hears its name, but for those who do, staying safe deserves real attention.
You’ll rarely find dramatic headlines about MPS causing chaos at factories or in households. Still, digging into scientific data, this compound does not count as harmless. Skin can get irritated after direct contact. Eyes sting and water up. If you breathe in the powder, your throat and nose react fast. On top of that, the sulfur group in its structure creates an odor that often bothers people after a spill.
More than once, people learned the hard way that repeated or prolonged exposure brings issues. Asthma-like symptoms sometimes show up with repeated inhalation. There’s some evidence—though not dramatic—linking mercaptans to headaches and nausea if you stay around high concentrations. Most data links direct exposure to acute symptoms, but so far, nobody’s rushing to declare it a cancer risk. The problem lies more in irritating the skin, lungs, and eyes sharply in a short amount of time. That makes it a challenge for workers, especially without solid safety gear.
I spent time in a small lab a few years back, and every chemical—common or obscure—demanded care. Workers there wore gloves, goggles, and even, sometimes, face masks. Not everyone understood why all the precautions were needed. The story with MPS really echoed that point. You can’t trust appearance. Some chemicals hide their risks, and MPS belongs in that group. It looks like any other white powder. But if it drifts onto your skin or you accidentally touch your eye, it immediately teaches you the pain behind ignoring protocols.
Manufacturers of MPS include pages of warnings on safety data sheets: always use in a well-ventilated spot, avoid breathing dust, and never skip personal protective equipment. Overlooking these directions, either out of laziness or habit, opens up workers to trouble. I remember cases of minor burns and rashes among new lab staff. Thankfully, fast washing helped, but not everyone got off so easily.
You won’t banish risk altogether, but honest training with updates works wonders. New hires need guided practice—not just a pile of paperwork to sign. Good-quality gloves, splash-proof goggles, and a real-fit tested mask form a sturdy line of defense. Regular ventilation upgrades save lungs, as does sweeping up dust before it piles up. Emergency wash stations already proved their worth more than once.
Big-picture, clear labeling and steady risk communication mean a lot. That sense of watchfulness sticks, even outside of classrooms or labs. Respecting chemicals like MPS in daily routines helps everyone walk out healthy at the end of the day.
Sodium 3-mercaptopropanesulphonate doesn’t roll off the tongue, but if you work in electroplating, pharmaceuticals, or specialty chemicals, you probably know it better than your favorite lunch spot. Most places list its purity around 98–99%, calling that "analytical" or "technical grade." That figure isn't just there for show. Purity in a chemical like this tells you a lot about how well your end product will perform, how safe the process will be, and frankly, how many headaches you’re in for in the lab or on the shop floor.
Impurities—think inorganic salts, moisture, and leftover reactants—are never spelled out on the front of the drum, but they love to mess with your results. Too much sodium chloride sneaking in will weaken your brightener in plating baths. Not enough purity and your pharmaceutical batch might fail an entire set of QC tests. Even a fraction of a percent, say 0.5%, matters when hundreds of kilograms are involved. I’ve seen a production halt for three days because a supplier sent 96% purity stock, and the only sign of it was a dull finish on copper plating. It's one of those real-world reminders that a small detail listed on a spec can cause downtime, wasted product, and extra trips to the QA office.
For those used to seeing documentation, the certificate of analysis for this product usually spells out: assay (main content), moisture, heavy metals (measured as lead), iron, and sometimes chloride. High-purity sodium 3-mercaptopropanesulphonate should read as follows:
Some labs run further checks on related substances or specify residual solvents if the material enters sensitive applications like injectable drugs. Lower grades exist, but trimming the purity saves cents upfront and often costs dollars later in failed batches and waste disposal.
If you’re in charge of a plating line or process development, one bad batch can mean scrapping thousands of dollars of inventory. An old colleague once said, “It’s the invisible stuff that bites you.” So, blind trust in supplier stats is a risk. Good labs verify incoming lots with a titration or HPLC readout to check, even if the previous twenty shipments were flawless. Companies with ISO certification or working to FDA standards face even tighter restrictions. Miss a trace impurity and you're looking at batches with unexpected color, solubility problems, or—worse—a recall notice.
People who buy sodium 3-mercaptopropanesulphonate for regular use do better with strong paperwork. Third-party audit reports, a transparent chain of custody, and an experienced supplier all matter. Site audits are not just box-ticking—they reveal if a company actually segregates their raw materials or cleans equipment between runs.
Hunting down higher-than-needed purity doesn’t always pay. If a process only uses a small amount and isn’t sensitive to by-products, 98% might be enough. But for the risk-averse in pharmaceuticals or electronics, always pay a little more for that extra percent. It saves a pile of frustration, keeps processes running, and means fewer late nights at the plant.
| Names | |
| Preferred IUPAC name | Sodium 3-sulfanylpropane-1-sulfonate |
| Other names |
MPS 3-Mercapto-1-propanesulfonic acid sodium salt 3-Mercaptopropane-1-sulfonic acid sodium salt Sodium 3-mercapto-1-propanesulphonate 3-MPS Sodium propanesulfonate mercapto |
| Pronunciation | /ˈsəʊdiəm θriː mɜːˌkæptəʊˌprəʊˌpeɪnˈsʌl.fəˌneɪt/ |
| Identifiers | |
| CAS Number | 17636-10-1 |
| Beilstein Reference | 1723518 |
| ChEBI | CHEBI:62610 |
| ChEMBL | CHEMBL41518 |
| ChemSpider | 20536 |
| DrugBank | DB11371 |
| ECHA InfoCard | 100.034.993 |
| EC Number | 243-091-9 |
| Gmelin Reference | 83708 |
| KEGG | C05533 |
| MeSH | D017628 |
| PubChem CID | 23669124 |
| RTECS number | TH6370000 |
| UNII | 261LST0CWR |
| UN number | UN3436 |
| Properties | |
| Chemical formula | C3H7NaO3S2 |
| Molar mass | 170.18 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.37 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.6 |
| Basicity (pKb) | 9.21 |
| Magnetic susceptibility (χ) | -37.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.497 |
| Dipole moment | 6.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 196.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V03AB33 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P280, P302+P352, P305+P351+P338, P312 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Lethal dose or concentration | LD50 Oral Rat 2890 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | NIOSH: **WE8375000** |
| PEL (Permissible) | Not established |
| REL (Recommended) | 35 mg/m³ |
| Related compounds | |
| Related compounds |
3-Mercaptopropionic acid Methanesulfonic acid Sodium methanesulfonate Sodium thioglycolate Sodium 2-mercaptoethanesulfonate |