Sodium 3-chloro-2-hydroxypropanesulphonate didn’t pop up overnight. The compound gained interest in the latter part of the 20th century when chemists started chasing specialty chemicals that could improve surfactant production and industrial processes. Back then, chemical engineers spent a lot of time trying to solve issues in the textile and pharmaceutical sectors, especially problems tied to stability and reactivity under routine manufacturing conditions. After years of work on related chlorinated molecules, researchers saw real potential in this three-carbon sulphonate, both as a standalone and as an intermediate for more complex molecules. The chemical’s specific structure offered manufacturers a way to streamline synthesis and skip over complicated machinery or hazardous reagents. With this shift, industrial chemists found a workhorse with enough versatility for further development.
Sodium 3-chloro-2-hydroxypropanesulphonate falls into the category of halogenated hydroxy sulphonates, and people working in labs recognize it by its white, crystalline appearance and high solubility. Chemical suppliers now ship it worldwide to serve businesses focused on textiles, detergents, and specialty chemicals. Folks who spend their days formulating products that need robust surfactants or stable intermediates have grown attached to this compound for its predictable behavior, especially in environments where reliability matters more than anything else. Companies list the product under several trade names, but the main draw always comes back to its chemical backbone and how it fits into an array of reactions without much fuss.
Its molecular formula, C3H6ClNaO4S, hints at reactive sites that offer both flexibility and a fair share of safety considerations. The solid, odorless crystals dissolve quickly in water, allowing users to work with concentrated stock solutions or gentle dilutions depending on application. The melting point sits well above typical room temperatures, making storage simple if containers stay dry. This compound resists breakdown under most ambient storage and working conditions, resisting oxidation at room temperature. The sulfate group brings notable hydrophilicity, while the chloro substituent delivers reactivity that launches more specialized syntheses. The low volatility and modest corrosivity mean shipping regulations focus on stability rather than outright hazardous classification, a relief for procurement teams striving for hassle-free imports.
Material coming out of plants meets strictly defined technical specifications. Purity typically hovers above 98%, with less than half a percent of residual byproducts. Specification sheets list moisture content, particle size distribution, and metal impurities, all of which vendors document for traceability and regulatory compliance. Bulk drums often carry GHS-compliant labeling, including hazard pictograms for skin and eye irritation and guidance on first-response protocols if accidental exposure occurs. Alongside, there’s clear advice on shelf life—manufacturers usually guarantee stability for two years under standard storage, with batch numbers and manufacturing dates easy to trace should questions arise. Down the chain, end-users check these specs regularly before introducing new batches into production, since cross-contamination would ruin downstream polymers or pharmaceuticals.
Lab workers prepare sodium 3-chloro-2-hydroxypropanesulphonate through a straightforward reaction between epichlorohydrin and sodium bisulfite. By controlling conditions closely—especially temperature and addition rate—producers prevent side reactions that yield undesirable isomers or excessive tar. After reaction, the product crystallizes out of the aqueous phase, and filtration removes unwanted solids. Any colored impurities get separated during washing and drying steps. In industrial-scale runs, continuous flow reactors help tighten tolerances and minimize worker exposure, helping plants operate with fewer interruptions or manual interventions.
The sulphonate and hydroxy groups offer chemists multiple routes for further reaction. Etherification, esterification, and nucleophilic displacement all open doors to custom molecules. This compound frequently serves as a building block for high-performance detergents and textile auxiliaries. The chloro substituent, in particular, brings unique reactivity for introducing new groups at the three-carbon bridge, and this makes the compound a mainstay in research projects looking to tailor-make surfactants or graft specialized appendages for pharmaceutical intermediates. The molecule’s stability under mild conditions makes it easy to control process yields without sacrificing purity, streamlining quality control downstream.
Chemists use a handful of names to identify this compound. It shows up in older literature as sodium epichlorohydrin sulphonate and pops up on chemical supplier lists under terms like sodium 3-chloro-2-hydroxypropane-1-sulphonate. The trade often sticks to abbreviations or catalog numbers, but whatever the lingual approach, buyers and researchers almost always home in on the unique combination of airway-friendly chlorinated hydroxy and the unmistakably robust sulphonate anchor. This helps avoid cross-talk with similar but less versatile molecules.
Handling sodium 3-chloro-2-hydroxypropanesulphonate means taking practical care, just like with most industrial chemicals. Folks in shipping and warehouses wear nitrile gloves and safety goggles not out of habit, but out of necessity—the substance can trigger mild skin and eye irritation if spilled. Proper ventilation keeps dust down, and industrial vacuum systems scoop up escapees fast to ward off respiratory complaints. Routine air testing, spill response kits, and annual safety training courses ensure neither complacency nor carelessness creeps into workplaces, especially in plants running round-the-clock shifts. Wastewater and byproduct handling follow official local and international guidelines, which reduces the risk of environmental release or community exposure.
Most users encounter sodium 3-chloro-2-hydroxypropanesulphonate in textile and surfactant development. Factories working on softeners, dyeing assistants, and specialty cleaning agents lean heavily on the compound for its reliable reactivity and compatibility. Labs working on pharmaceutical precursors appreciate the molecule’s ability to serve as a bridge for more complex active ingredients. In water treatment facilities, this chemical sometimes appears in the fine-tuning of coagulant aids and antiscalants. Recent years saw demand increase in electronics and semiconductor sectors, where purity and predictable performance matter more than bulk cost.
Academic chemists and industry teams keep expanding the book on sodium 3-chloro-2-hydroxypropanesulphonate. Over the last decade, research efforts dug into new applications, like using the compound as a precursor for tailor-made polymers and functionalized resins. Analytical chemists lean on high-resolution spectroscopy and chromatography to analyze trace impurities and confirm reaction pathways. In turn, process engineers chase ways to improve yield or cut down on water and energy inputs, promoting both efficiency and sustainability. Collaborations with regulatory laboratories help define clearer guidelines for additives, and open data sharing ensures others in the field can spot issues or opportunities early.
Scientists study environmental and biological effects closely. Toxicologists track acute and chronic exposure outcomes, focusing both on direct handling and long-term environmental buildup. So far, the compound’s moderate toxicity profile means standard precautions work as they should, provided operators respect dosage rules and use personal protective equipment. Researchers aim to clarify breakdown products, investigating whether anything more persistent or harmful shows up during or after industrial use. Evidence points toward low bioaccumulation, but regulatory agencies advocate for continued long-term monitoring, given growing scrutiny on chemicals in water supplies and food chains.
The years ahead look promising for sodium 3-chloro-2-hydroxypropanesulphonate. Driven by shifts in regulatory expectations and industry demand for low-impact specialty chemicals, developers keep seeking greener synthesis routes and more recyclable packaging. As digital transformation touches even legacy chemical industries, process digitalization may help push quality, traceability, and safety standards higher. Smaller tech companies and university labs continue to mine this compound for fresh applications, building everything from novel surfactants to next-generation water treatment solutions. My own work with process optimization highlights how incremental gains in purity, yield, or waste mitigation translate to real-world profits or regulatory headroom. Every step forward—no matter how ordinary—helps strengthen the case for specialty chemicals that earn their keep both in workflow and in environmental stewardship.
Sodium 3-chloro-2-hydroxypropanesulphonate doesn’t exactly roll off the tongue, so most people outside chemistry circles barely give it a thought. This compound usually flies under the radar, yet it has built a quiet reputation in manufacturing and research. If you’ve ever used certain soaps, cleaning agents, or synthetic textiles, you’ve probably crossed paths with this chemical—whether you spotted its name on an ingredient list or not.
I spent a summer in my cousin's industrial laundry. Inside those sweltering walls, the cleaning crew dumped barrels of concentrated detergents into the giant wash systems. Many of these detergents depend on specialty chemicals to function well. Sodium 3-chloro-2-hydroxypropanesulphonate acts as a surface-active agent and helps break apart grime and oil, letting water wash them away. Without these kinds of additives, stains linger, and cleaning power drops.
The compound's chemical structure lets it work in both hard and soft water—unlike older ingredients bogged down by minerals. This advantage cuts costs for businesses while lowering the risk of lime build-up in pipes and machinery. Over time, that leads to fewer maintenance calls and longer-lasting equipment. Anyone who’s ever faced a rusty, gunked-up washer can appreciate this chemical’s role.
In textile mills, sodium 3-chloro-2-hydroxypropanesulphonate turns up frequently in bleaching and dyeing. Textile workers use it to highlight colors, keep fibers uniform, and make fabrics feel softer to the touch. On the leather side, tanneries apply similar chemistry to clean and soften hides. The chemical helps prevent uneven patches—something both luxury brands and bargain outlets want to avoid in finished products.
Getting the color right and making sure cloth or leather feels pleasant often determines value for both producer and customer. My own shirt drawer owes its bright whites and even colors to these behind-the-scenes chemical helpers.
With every useful chemical comes a responsibility to weigh safety. Reports show sodium 3-chloro-2-hydroxypropanesulphonate usually breaks down well in modern wastewater systems, which reduces worries about environmental accumulation. That doesn't give producers a free pass. Safety data sheets point to the importance of proper handling—no open vats, gloves required, solid training for anyone working near it.
The rise of green chemistry keeps pushing for alternatives made from renewable sources. Researchers have begun exploring plant-based surfactants and biodegradable cleaning molecules. Right now, compounds like sodium 3-chloro-2-hydroxypropanesulphonate still dominate, especially where cost efficiency and reliability figure heavily in the buying decision. Consumer pressure and tighter regulations may shift that balance over time.
Industry sometimes rushes headlong into solutions before every outcome is understood. Good stewardship means looking at each ingredient, tracking new research, and holding companies to high safety standards. Sodium 3-chloro-2-hydroxypropanesulphonate offers a useful tool for those making, cleaning, and coloring everyday goods, but it shouldn’t be seen as the end of the story. Responsible production and ongoing development in green chemistry will shape which chemicals we trust in the future.
In daily life, most people never hear about sodium 3-chloro-2-hydroxypropanesulphonate. Even in manufacturing circles, it hardly draws attention compared to more notorious chemicals. Some chemists recognize it under names like C3H6ClNaO4S, and it usually crops up as an additive or intermediate in detergent production, especially when companies tweak surfactant formulations. Still, like with many chemicals, safety means more than recognizing a label.
Cleaning, degreasing, or processing textiles, various sectors rely on chemistry that deals with tough stains or industrial byproducts. Workers may make direct skin contact, breathe in dust, or handle solutions with this sulphonate. As a rule, regulations ask for careful handling. Factories in Europe, North America, and parts of Asia need to follow safety data sheet instructions for each substance on their shelves.
Long chemical names don’t always translate to clear health risks. Researchers judge every ingredient by observing its action on the skin, eyes, lungs, and internal organs. For sodium 3-chloro-2-hydroxypropanesulphonate, the literature remains limited. Available studies—like those reviewed by the European Chemicals Agency (ECHA)—suggest low acute toxicity but report mild irritation if the substance stays on the skin or splashes into the eyes. Nobody wants to rub a raw spot with any cleaning agent. Proper gloves and goggles make all the difference in workplaces using this or similar surfactants.
In a chemical plant or a laundry additive factory, personal experience shows that many workers ignore gloves, especially during routine cleaning. After a few red patches or an unplanned eye splash, the lesson hits home. Skin rash or conjunctival irritation means the compound deserves respect, even if it doesn’t break down tissue like a strong acid. Industrial safety officers train employees to wash off spills, check labels for pictograms, and learn basic first aid. Clear, practical instruction helps more than just posting rules.
On the consumer side, most people face near-zero risk. Finished goods like detergents rarely leave active sulphonates sitting around in large quantities. Product regulations make sure only tiny, non-reactive traces survive the mixing process. Still, children sometimes swallow odd things, and people test spot removers on bare hands out of habit. If there’s ever redness or stinging, rinsing the area under running water remains the fastest fix. For a chemical like this, water wins most races.
Transparency helps everyone. I’ve seen chemical companies shift from terse data sheets to clearer warnings, marking out everything from “causes irritation” to “may cause an allergic skin reaction.” This helps workers and buyers decide just how much risk to accept. For sodium 3-chloro-2-hydroxypropanesulphonate, direct, prolonged contact can cause minor surface problems. Manufacturers and safety regulators suggest minimizing contact, wearing protective clothing, and storing the material securely.
Most harm comes from skipping simple safety habits. Respect for chemicals—however obscure their names—protects workers and consumers. Ongoing monitoring, updated safety leaflets, and easy access to washing stations reduce worry and harm, not only for this sulphonate but across the whole chemical industry.
Sodium 3-Chloro-2-Hydroxypropanesulphonate isn’t a household name for most people, yet plenty of folks in labs, factories, and research centers have reason to work with it. Chemicals like this one bring real benefits but can also create risks when ignored or handled poorly. I’ve seen talented lab techs and production workers get tripped up by overlooked details—simple temperature shifts, unlabeled containers, a little moisture sneaking in. Treating chemical storage seriously forms the start of a safe environment.
This compound does its best work kept away from dampness. Even a bit of humidity can alter its consistency, which messes with both measurement accuracy and handling. I’ve cracked open a poorly sealed container before and found stubborn clumps, which raises red flags in any setting where purity matters. Store sodium 3-chloro-2-hydroxypropanesulphonate in dry zones—think of a sturdy cabinet or dedicated chemical storage locker with a low-moisture climate. Labs use silica gel packs or other desiccants to draw out sneaky humidity, a trick that works almost anywhere.
Temperature control plays a big role too. Warm rooms accelerate unwanted reactions and can even break down the chemical over time. Storing this material at room temperature (20–25°C), away from direct heat or sunlight, keeps it steady and shelf-stable. Don’t leave it by a sunny window or near hot pipes. My time at a cement plant’s lab taught me how quickly heat ruins batches of even simple ingredients.
Sealed, chemical-resistant containers stop spills, evaporation, and contamination. Plastic drums with air-tight lids, or well-labeled bottles with screw-on caps, keep the compound secure between uses. Labels should state the full name, hazard info, and date of receipt. I once watched a colleague grab the wrong jar because “white powder” looked the same across three containers. Clear, bold labels remove guesswork for everyone, especially in busy environments where people rush.
Don’t mix this chemical in storage with acids, oxidizers, or volatile compounds. Even small leaks can cause unexpected reactions. At one facility, I saw a shelf collapse and create a puddle of two incompatible chemicals—only luck saved us from a bigger emergency. Give sodium 3-chloro-2-hydroxypropanesulphonate its own space. Use simple shelving charts or dividers so coworkers won’t stack unknowns on top by accident.
Store spill kits, nitrile gloves, goggles, and dust masks right by storage. Quick access shortens reaction time when accidents strike. A local metal plating shop drilled everyone on spill response and made those kits easy to find. Even fast, well-trained responses only work if tools sit close at hand.
Schedule regular checks of storage areas. Any sign of moisture, damaged lids, or clumping should prompt immediate cleanup and new containers. Keep records on who opens which container and when. Posting clear safety data sheets nearby gives everyone on shift a fast read on hazards and handling advice.
Clear steps and shared know-how make all the difference. I’ve seen real harm avoided by respecting the details: dry air, safe shelving, labeled containers, and teamwork. Every worker deserves a workplace that treats chemicals with a full measure of respect. Safe storage turns knowledge into protection, both for the people doing the job and the materials we depend on.
Anyone who has spent time in a lab or on a production floor knows that chemical shelf life can make or break an experiment or a batch. Sodium 3-chloro-2-hydroxypropanesulphonate sounds complex, but the issues surrounding it follow the same simple rule: quality fades when chemicals are exposed to the wrong conditions for too long.
I’ve seen plenty of warehouses and chemical storerooms where containers carry a printed shelf life, but real-world factors often tell a different story. For this compound, the shelf life usually sits around two years in sealed, original packaging under ideal storage. By “ideal”, I mean a cool, dry spot, away from sunlight and strong acids or bases. Temperature swings, moisture, and careless handling shave months off even the most optimistic expiry dates.
In my early days, I watched a batch lose potency in under a year just because someone stored it near a window. I learned quickly that a shelf-life guarantee only works on paper. Sulphonate salts in general like to absorb moisture, and clumping or yellowing means it’s time to replace them. The hydroxy and chloro groups tend to break down with water or heat, leading to impurities that nobody wants, especially if purity impacts safety or end use.
I’ve met plenty of chemists who trust their noses and eyes more than the date stamp. Discoloration and strange smells offer better warning than any paperwork. The best labs I’ve visited track humidity and temperature, using silica packs or desiccators, and keep strict inventory rotation. Small things such as checking for caked or lumpy powders do more to preserve quality than most realize.
Compounds past their prime can act unpredictably, especially in pharmaceutical or research applications. Impurities can throw off results, causing researchers to chase false leads. In larger manufacturing settings, degraded material might cause safety issues or expensive recalls. It’s more than just numbers on a label—it’s about trust in results and products. Anyone storing this chemical for commercial use should plan on routine analysis, not just expiration tracking.
One trick that’s stood the test of time: batch documentation and occasional retesting. Some manufacturers test stability under various stress conditions, but real-life settings often differ from test environments. Keeping smaller containers, tightly sealed, and only opening as needed helps limit exposure. In busy labs, training everyone on proper resealing and wiping down containers matters as much as big investments in climate control.
Regulations often follow straight logic: discard anything beyond its stated shelf life. I suggest more proactive measures. A regular schedule for inventory checks, integrating software that logs conditions and usage, cuts down on surprises. For those unsure about the integrity of an old batch, most suppliers are happy to guide on retesting protocols or offer replacements.
Trust in a chemical’s shelf life starts in storage and ends with vigilance. If results or safety depend on sodium 3-chloro-2-hydroxypropanesulphonate, treating it with the respect you’d give any sensitive ingredient pays dividends. No flashy technology replaces basic care and common sense—store it right, check it often, and your shelf-life concerns shrink fast.
Handling chemicals has always demanded respect. I recall walking into an industrial storeroom as a young tech and the air itself seemed to be taking notes on who paid attention. Sodium 3-chloro-2-hydroxypropanesulphonate isn’t the sort of compound you leave out or toss into a box with everything else labeled "miscellaneous." Its structure means it can react with plenty of common substances. Careless handling invites spills, health problems, or fires.
Familiar precautions make a difference. This isn’t something you pour or scoop without gloves or splash goggles. I’ve seen tough workers pay the price for skipping protection—the red eyes, the skin burns, the visits to first aid. Basic gloves, aprons, eye shields, and a working exhaust system never go out of style here. Skin contact and inhalation cause real issues with this stuff, so keeping it off your body and out of your lungs always comes first.
Every container holding sodium 3-chloro-2-hydroxypropanesulphonate ought to offer more than just a lid. Any moisture leaks or air exposure set up caking, clumping, or sometimes slow, tricky reactions. I learned early in my career that even the angle a drum rests at can make it easy or hard to open without a spill. Tightly sealed, labeled drums kept off concrete by pallets avoid a lot of heartache. Storing chemicals away from heat sources, sunlight, or the usual suspects—acids, bases, organic solvents—cuts down on incidents.
Sometimes the old metal drums look bulletproof, but I’ve seen them rusting before their time. Polyethylene or lined barrels survive longer. Expiry dates matter because the compound can break down, and weakened material isn’t worth the risk. Even on a busy day, double-checking those labels before opening keeps mistakes off the logbook.
Anyone moving this compound knows the road isn’t the only place things go sideways. Warehouse ramps ice over, forklifts drop stuff, weather changes plans. I once watched a shipment go wrong simply because the drums weren’t strapped tight—the noise in the truck should have tipped us off. Use spill-proof, upright containers secured to the trailer or truck bed. For short moves, even a handcart means nothing if the load isn’t strapped in.
Local laws rarely get ignored in big operations, but smaller outfits sometimes cut corners. Transporters carry material safety data sheets, accident kits, and at least one worker who knows emergency procedures. If a drum breaks open, that first five minutes will determine whether problems get contained or turn into an environmental report and a hefty fine. Accessibility of eyewash stations and neutralizing material can mean the difference between a brief cleanup and a medical emergency.
Some companies run tight ships but others operate with aging equipment or old habits. Regular training refreshers, using modern storage materials, and investing in better leak detection give real-world protection. Rather than seeing guidelines as red tape, I grew to think of them as the handrails that keep workers out of the ER. Those changes build trust within a team and cut down on “What went wrong?” meetings. That’s not just compliance; it’s prevention with a human touch.
People forget that the stakes include personal health, local water supplies, and company reputation. Handling and transport practices deserve regular checks by folks who know what can go wrong—not just what’s on the paperwork.
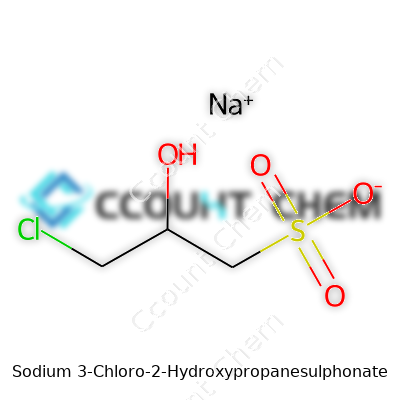

| Names | |
| Preferred IUPAC name | Sodium 3-chloro-2-hydroxypropane-1-sulfonate |
| Other names |
Sodium 1-chloro-2-hydroxypropane sulfonate CHPS-Na Sodium (2-hydroxy-3-chloropropyl)sulfonate |
| Pronunciation | /ˈsoʊdiəm θri ˈklɔːroʊ tu haɪˈdrɒksiˌproʊˈpeɪnˌsʌlˈfəneɪt/ |
| Identifiers | |
| CAS Number | “126-83-0” |
| 3D model (JSmol) | `C[S@H](Cl)CO[S](=O)(=O)O` |
| Beilstein Reference | 1718731 |
| ChEBI | CHEBI:91251 |
| ChEMBL | CHEMBL1838312 |
| ChemSpider | 15830854 |
| DrugBank | DB09136 |
| ECHA InfoCard | 03c67d43-e9bb-45e3-bb44-06e1c5755402 |
| EC Number | 215-396-4 |
| Gmelin Reference | 88257 |
| KEGG | C18907 |
| MeSH | D020132 |
| PubChem CID | 12521 |
| RTECS number | TN0175000 |
| UNII | B9QXM53JB8 |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C3H6ClNaO4S |
| Molar mass | 202.60 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.6 g/cm³ |
| Solubility in water | Soluble |
| log P | -3.2 |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | 12.98 |
| Magnetic susceptibility (χ) | -53.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.475 |
| Dipole moment | 5.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | Std molar entropy (S⦵298) of Sodium 3-Chloro-2-Hydroxypropanesulphonate is 334.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1342.8 kJ/mol |
| Pharmacology | |
| ATC code | A16AX05 |
| Hazards | |
| Main hazards | Irritating to eyes, respiratory system and skin. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 122 °C |
| Lethal dose or concentration | LD50 Oral - rat - > 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | WM8520000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/m³ |
| Related compounds | |
| Related compounds |
2-Chloroethanesulfonic acid Sodium isethionate 3-Chloro-2-hydroxypropyl methanesulfonate Chlorohydrin Sodium 2-hydroxyethanesulfonate Epichlorohydrin Sodium chlorosulfonate |