Chemistry has never stood still. Pioneers in surfactant and specialty chemical research have always chased new ways to tweak molecules, not just for efficiency, but to answer changing needs in detergents, water treatment and materials science. Sodium 3-(Allyloxy)-2-hydroxypropanesulphonate emerged through decades of trial and error, where scientists kept an eye out for sulfonated, water-soluble agents that would not break down under tough industrial conditions. I’ve read accounts from the 1980s where researchers started recognizing the benefits of allyl side chains and free hydroxyl groups, especially in the field of reactive surfactants and as functional additives. Chemists started using selective sulfonation techniques, aiming for molecules that provide excellent wetting, dispersing, and electrostatic-stabilizing properties. Without that persistence and curiosity, markets would have stuck with conventional alkanesulfonates, lagging behind in both sustainability and performance.
In real-world terms, Sodium 3-(Allyloxy)-2-hydroxypropanesulphonate acts as a specialty surfactant that finds a home in industrial cleaning, electroplating, polymer synthesis, and even concrete plasticizers. Its structure blends the reactivity of an allyl group with polarity from sulfonate and hydroxy functionalities, which gives it a unique edge. I’ve seen that the balance of hydrophilic and hydrophobic sites allows it to perform where traditional surfactants can’t handle high electrolyte conditions or require rapid reactivity for cross-linking and polymerization.
The product typically appears as a white to off-white powder or sometimes as a concentrated liquid solution. It dissolves easily in water, forming clear, stable solutions without visible residue. With a molecular weight around 216 g/mol and strong ionic character from the sulfonate group, sodium 3-(allyloxy)-2-hydroxypropanesulphonate handles extreme pH and temperature swings better than many conventional agents. Its pH in aqueous solutions sits in the mildly alkaline range, fitting well with most cleaning formulations and industrial baths. Chemical resilience sets it apart. The presence of an allyl ether enables participation in radical polymerizations and other modifications that simpler surfactants can’t pull off.
Factories providing this product often highlight purity, sodium content, water content, and trace impurities. I’ve seen spec sheets listing sulfonate content in the 38–42% range, alloyed with a sodium content typically close to theoretical levels. Labels must spell out CAS number 132971-51-6, major hazards, recommended personal protective equipment, and instructions for storage in cool, dry conditions away from direct sunlight or acids. REACH registration and reaction hazard guidance have become standard, especially for export markets. I always recommend checking certificates of analysis for each production batch, since minor differences in color or moisture can influence performance in sensitive formulations.
The synthesis unfolds in a few distinct steps. Most methods start with allyl glycidyl ether, which reacts with sodium bisulfite in aqueous medium. Stirring under controlled temperature and pH encourages nucleophilic addition, selectively opening the epoxide ring to give the hydroxypropanesulphonate backbone. Some routes use sodium thiosulfate with subsequent oxidation. The key challenge lies in keeping side reactions to a minimum. I’ve heard of labs dealing with incomplete conversion or unwanted polymerization unless reaction conditions are precisely dialed in. After the main reaction, filtration and removal of inorganic byproducts polish up the end product. Several suppliers run continuous processes, cutting waste and boosting batch-to-batch consistency.
Beyond simple use, this molecule becomes a versatile building block. Chemists leverage the allyl group for further functionalization—think graft polymerization or Michael additions that introduce new performance features. In polymer chemistry, it earns its keep as a monomer or reactive surfactant, creating hydrogels, superplasticizers or specialty copolymers with precisely tuned properties. The sulfonate group ensures strong ionic interactions, while the hydroxyl opens the door to potential esterifications or further coupling. This sort of modularity is rare, but invaluable when designing tailored solutions for industries as diverse as oil recovery or antistatic coatings.
People working with specialty chemicals know that names can confuse more than they clarify. This compound sometimes travels under the names Allyloxy-Hydroxypropanesulfonic Acid Sodium Salt, Sodium 3-(2-hydroxy-3-allyloxypropyl)sulfonate, or AHS-Na. Some older literature uses obscure trade designations, so double-checking CAS numbers remains good practice.
Handling any sulfonate or allyl compound calls for care. Eye and skin irritation is possible with prolonged contact. Gloves, goggles and protective clothing rank as essential gear whether you’re tinkering at the bench or blending at scale. Current safety data suggests very low acute toxicity by oral or dermal routes, and the material does not bioaccumulate or persist in the environment, which already makes it a step ahead of older surfactants. Still, spills near sensitive ecosystems or water treatment plants should get immediate clean-up. The product passes GHS/CLP standards in Europe, and North American imports require comprehensive labeling covering decomposition, fire hazards (it forms sulfur oxides if burned), and safe disposal methods. On-site training for staff cuts down risks, especially for new hires who may underestimate how fast a chemical can splash or how easily dusty solids can be inhaled.
I keep seeing sodium 3-(allyloxy)-2-hydroxypropanesulphonate pop up in places you might not expect. Concrete admixtures gain flowability and strength. Water-soluble resins and coatings benefit from improved dispersion. Metal finishing baths, such as those used for electroplating, rely on it to maintain bath stability and surface quality. In textile processing, it works as an anti-redeposition and leveling agent, ensuring that dyes distribute evenly during tough dyeing cycles. I have even seen patents mentioning it as a co-monomer in synthetic hydrogels and as a stabilizing agent in nanotechnology and microelectronics development. Its broad scope boils down to a mix of reliable performance, chemical resilience, and easy incorporation into existing processes.
Universities and R&D labs have been nudging this molecule into new territory. Eco-friendly construction materials look to slash cement content using smarter plasticizers; sodium 3-(allyloxy)-2-hydroxypropanesulphonate offers a promising route, thanks to its strong interaction with cement particles and water. Resin chemists blend it into emulsions and latexes, tuning water retention and film development for paints and adhesives that outperform older benchmarks. Researchers are also probing new synthetic pathways, like using biobased allyl glycidyl ether or greener sulfonation agents, to reduce environmental impact. I have spoken to chemists exploring clever modifications—introducing phosphonate or carboxylate groups for hybrid polymers showing promise in battery electrolytes and advanced membranes.
Toxicity remains a top concern, especially for large-volume industrial chemicals. Tests using fish, daphnia, and algae point to relatively low aquatic toxicity. Biodegradation studies demonstrate partial breakdown in natural waters, aided by microbial populations. Chronic exposure does not trigger significant mutagenicity or long-term bioaccumulation in mammals. Workers need to observe basic protective measures, but the compound’s lack of persistent toxicity stands out when compared to classic alkylphenol sulfonates or other outdated surfactants. Distribution in the environment doesn’t seem to concentrate in soil or food chains, lowering long-run ecological risk.
Demand for versatile, sustainable chemicals will keep rising. Sodium 3-(allyloxy)-2-hydroxypropanesulphonate has already stepped beyond its original role in cleaning and dispersing. Polymer and materials science will likely see even more tweaks to its core structure, such as introducing new functional groups or partnering it with biopolymers. More efficient, less wasteful synthesis can push the carbon footprint down further. Regulatory pressure may nudge suppliers to minimize impurities and guarantee batch purity for medical and food-contact applications. Companies seeking a competitive edge in everything from additive manufacturing to green building materials will watch this compound closely, not just seeking drop-in replacements, but whole new performance landscapes.
Digging into technical-sounding chemicals often feels intimidating, but sodium 3-(allyloxy)-2-hydroxypropanesulphonate carries a simple job: it keeps things from clumping, sticking, or settling in products we use every day. This substance works as a dispersant, meaning it helps mix particles smoothly throughout liquids. In paint, detergents, and even cement, this chemical keeps materials even and easy to apply or pour.
Any homeowner knows a good coat of paint can change the mood of a room. But that solid finish needs more than just color—it depends on chemicals like sodium 3-(allyloxy)-2-hydroxypropanesulphonate. Pigment particles float evenly throughout the can and coat the wall without streaks or patchiness. Too much clumping, and paint loses its appeal. Dispersants step in, letting paint flow across the surface evenly. This chemical also helps keep painting easy for both professionals and DIYers, cutting down on wasted product and frustration.
Laundry and dishwashing detergents often include this sulphonate compound for similar reasons. Water alone would leave dirt and leftovers clinging to clothing or dishes. Dispersing agents break down grime and help it wash away instead of redepositing. This chemical's structure works in both hard and soft water, supporting “clean” as a real result instead of just a marketing slogan.
Walk past any construction site, and the air fills with dust and the hum of concrete mixers. Sodium 3-(allyloxy)-2-hydroxypropanesulphonate plays a less visible but essential part in these environments. Construction crews use additives like this in concrete mixtures to keep particles evenly spread out, which helps the material pour and set more predictably. These agents also help builders shape and finish projects faster, using less water and energy in the process.
Working with chemicals means paying close attention to health and environmental impact. According to published safety data, this compound doesn't hang around in the environment for long. It biodegrades quickly, leaving little behind compared to other more persistent chemicals. That doesn't mean anyone gets careless—workers still use gloves and eye protection—but compared with older additives, this substance tends to support safer workplaces and lower ecological risk.
Scientists often look for ways to improve the goods we rely on, and dispersants are no exception. Efforts focus on making them even safer and more sustainable. Whether it’s finding sources from plants or by tweaking formulas to perform better at lower doses, innovation continues. Companies might also turn to public data to support claims about their products, showing they meet today’s standards for both safety and environmental impact. As customers and regulators push for cleaner, safer products, sodium 3-(allyloxy)-2-hydroxypropanesulphonate finds itself under study—and sometimes, praise—for keeping us cleaner, safer, and a little more colorful.
If you’ve ever read a label on a bottle of cleaning agent, you already know – handling chemicals isn’t just book knowledge. Over the years, I’ve seen small mistakes lead to injuries, missed work days, and expensive cleanups, all because safety took a back seat. People can forget how just one splash or sniff in the wrong place can change a regular shift into a trip to the ER. It’s not about paranoia, it’s about respect for what these substances can do.
The gloves, goggles, long sleeves, fitted masks – nobody wears this stuff for style points. Think about nitrile gloves. They block a surprising range of chemicals, but pick the wrong material or size, you’re rolling the dice with your skin. Eye protection shields sensitive eyes from accidental sprays. I still remember a coworker splashing a caustic degreaser and scrambling to the eyewash station in seconds. If he didn't have his goggles on, that story might have ended with permanent damage.
It only takes a little exposure to certain fumes to cause serious health issues. Proper ventilation isn’t just a box to check; it keeps lungs from soaking up vapors that can burn, dizzy, or poison you. Even now, seasoned pros crack a window, use a fume hood, or exhaust fan before mixing chemicals. Fresh air is a friend in these environments.
Mixing the wrong items, like bleach and ammonia, risks creating toxic gas. Nobody wins in that scenario. Before opening a chemical container, check the label. If it’s faded or missing, don’t touch the stuff—misidentification leads to big mistakes. Manufacturer safety sheets (SDS) might not make for fun reading, but they explain hazards, what gear matches up, and what to do if something goes wrong. Reading up beforehand beats flipping through instructions during a crisis.
Stuffing chemical containers onto any available shelf might save time now, but causes headaches down the line. Some chemicals react with others if they get loose. Flammable liquids, corrosives, oxidizers—each belong in their own safe spot, away from sunlight, heat, and curious hands. Locked cabinets and clear labels matter for safety and for tracking inventory.
Spills don’t always make the nightly news, but they’re common behind the scenes. Having a spill kit – absorbent pads, neutralizers, a clear evacuation plan – keeps panic to a minimum. Training helps as much as equipment. People who know what to grab and where to go protect themselves and their coworkers every time.
Proper training beats out fancy gear. People who understand chemical risks act smarter from the start. They keep hands away from faces, avoid mixing chemicals in coffee mugs, and clean up before eating. No shortcuts, just habits drilled in through experience and reminders on the job. This knowledge saves lives and careers.
Handling chemicals safely improves with each lesson learned. Reporting near-misses, updating protocols, and sharing what went wrong means everyone gets better at avoiding trouble. Companies that encourage open discussion around mistakes create workplaces where people look out for each other.
Each precaution comes from hard lessons learned. Every label, glove, and safety drill builds a culture where safety grows from real experience, not just out of rulebooks. Understanding why these steps matter protects more than physical health – it covers livelihoods and keeps teams running strong.
I have worked in labs and small businesses where even a small slip in storage made a big mess. Think of food, medicine, or any sensitive product—how it sits on a shelf isn’t just a rule on a label. It’s a genuine piece of food safety, public health, and product value. Take a supplement or ingredient product. If that jar lives in humidity or heat, you don’t just lose time and money. You often endanger health.
In 2022, the US Food and Drug Administration recalled hundreds of thousands of bottles of medicine. Not bad manufacturing. Not a problem with ingredients. Storage failed. Warehouse temps spiked. Bottles warped. People downstream—from patients, to parents—got products that did not work as promised. Something as avoidable as bad shelf conditions created a huge ripple.
The main enemies come down to moisture, temperature swings, and light. Too much humidity, and powders clump or spoil. Tablets grow soft, mold creeps in, or even chemical breakdown starts far before the “best by” date. Heat speeds up these failures. I’ve seen nutrition products packed for 18 months of shelf life looking and smelling wrong after two months in a sunlit storeroom.
Some folks forget about light. Sun or bright indoor lights can bleach packaging and break down sensitive ingredients. Even a clear plastic jar can let in enough sun to weaken valuable nutrients.
Based on experience and expert sources—from the CDC, FDA, and decades of manufacturer advice—proper storage for most supplements, medications, and food products asks for a dry, cool, and dark space. Temperatures between 15°C and 25°C (59°F–77°F) cover the sweet spot for most household products. Humidity below 60% brings even greater safety. These don’t just sound good—they match guidance published by regulators and scientific review.
Crowd tight spaces and that airflow drops, which raises both risk of moisture and temperature pockets. I always encourage staff to leave some room for air to move on shelves, to put heavier or older stock in front so nothing lingers in the back.
People sometimes skip over the simple “keep tightly closed” warning. Leaving a lid loose draws in moisture and contaminants. Labeling may look dry and boring, but it works.
Some businesses turn to better packaging—think foil packs for supplements or amber bottles for light-sensitive liquids. Remote sensors, now affordable even for a small business, send real-time alerts if temps or humidity slip out of range. Even a basic thermometer and hygrometer on a shelf can reveal trouble before stock goes bad. For consumers, a kitchen cupboard away from the stove and window solves most problems.
Mistakes do happen. In hectic work, it’s easy to store something “just for now.” But in truth, small changes build habits and protect the folks at the end of the line. Storage isn’t just a checklist—it’s an act of care for everyone who eventually uses the product.
Sodium 3-(Allyloxy)-2-Hydroxypropanesulphonate sounds like something cooked up in a lab a world away from everyday concerns. In truth, it plays a role in a number of industries, especially when a strong sulfonate surfactant is needed. The way this compound interacts with water gets top billing here.
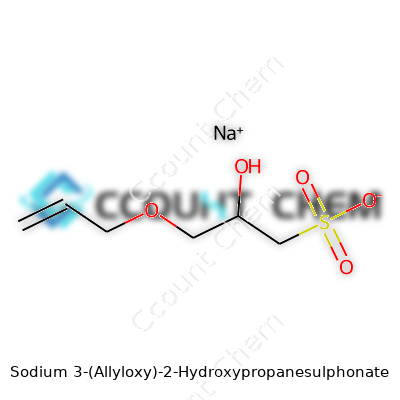
The structure gives away a lot. The sulphonate group bonded with sodium forms the backbone for water solubility. Sulfonated materials like this react well with water because the sulfonate group itself draws water molecules to it, no matter the temperature. That friendly sodium ion seals the deal; sodium salts, especially of sulfonic acids, dissolve well in water. If you’ve worked with detergents or water treatment chemicals, you might have seen related compounds perform the same trick—dissolving into clear solutions without fuss.
Solubility isn’t just a trivia point in chemistry class. In the real world, a water-soluble chemical opens up possibilities in cleaning, construction, and even cosmetics. From personal experience working with water treatment specialists, a chemical’s ability to dissolve quickly without leaving residue makes or breaks a project. Water-soluble chemicals also simplify dosing and mixing. Imagine mixing a cleaning solution: you want your surfactant to dissolve rapidly and completely. Any leftover grit or cloudiness means it’s not doing its job, and you’re wasting money.
Textbooks like “Surfactants in Water-Based Systems” spell it out plainly—sulfonate groups behave like magnets for water, especially when partnered with sodium. Lab data shows sodium 3-(allyloxy)-2-hydroxypropanesulphonate dissolves in water at concentrations well above what’s required for most uses. For anyone who has made solutions using sodium-based surfactants, the process goes smoothly. There’s no need for extra heat or mechanical help. The solution stays stable, too, without separating out over time.
Industries value ease of use and predictability. Take textile processing. Clear, quick-dissolving surfactants keep water cycles short and equipment free of buildup. In municipal water treatment, solubility translates to faster, more reliable results. My time consulting for municipal water managers led me to respect these small differences. Soluble chemicals save on labor, cut waste, and make operations easier across the board.
Though dissolution comes naturally to this compound, problems sometimes crop up. Impure water, cold conditions, or poor mixing can cause lumps or slow dissolution. If this happens, addressing water quality or adding gentle stirring usually solves things. Companies have started packaging such chemicals in pre-dosed pouches that dissolve directly in water, reducing human error and protecting staff from exposure. I’ve set up systems where these pouches performed flawlessly, cutting setup time and chemical waste.
Working hands-on with a wide range of water treatment chemicals has proved one thing to me: solubility often tells you most of what you need to know about how a surfactant will perform. Whether you’re designing a new process or troubleshooting a stubborn tank, knowing your chemical will dissolve quickly and completely means fewer headaches down the road.
Everybody has seen warning labels on household cleaners, medications, or even foods. Sometimes, the words blend into the background of our daily routines. I remember scrubbing my kitchen with some powerful degreaser years ago. I didn’t think twice—until I caught my breath and felt my eyes sting. That moment snapped me awake to the real risks that come with handling chemicals, even ones sold at the corner store.
It’s not all paranoia. Chemical exposure lands millions in emergency rooms each year. According to the U.S. Centers for Disease Control and Prevention, accidental poisonings rank high, especially among kids. Scientists and health officials spend years researching how substances interact with our bodies, partly because the difference between something helpful and something deadly can hide in plain sight. Remember thalidomide? It began as a harmless-looking pill but left thousands with severe birth defects before the truth came out.
Hazards pop up in ways most people don’t expect. Some compounds irritate skin or eyes immediately—think of bleach or ammonia mixing, which throws off toxic fumes. Others sneak into lungs or bloodstreams with slow buildup. Heavy metals like lead and mercury once seemed useful in paints and thermometers until long-term, low-level exposure ended up causing serious brain and kidney damage. To this day, children living near old industrial sites have higher risks of developmental issues.
Oversight on chemical safety has gaps. The Toxic Substances Control Act in the U.S. tries to keep harmful compounds out of reach, but the EPA lists tens of thousands under limited review. Companies sometimes release new formulas before enough independent testing has been done, and it’s people at home or workers in factories who deal with the consequences. Transparency suffers when patents or trade secrets block scientists from studying exact ingredients or processes.
One angle to keep in mind is the effect on workers. OSHA’s data shows that industrial employees, especially in cleaning, manufacturing, and agriculture, often develop asthma, rashes, or worse when safety protocols slip or equipment fails. Even with gloves and masks, repeated contact piles up. I met a janitor once who had to quit after years of handling strong disinfectants led to chemical burns on her hands.
There’s hope if we start, as communities and consumers, demanding greater transparency from chemical producers and government watchdogs. Independent bodies need louder voices in safety evaluations. Testing compounds before mass distribution must become the standard, not the exception. People benefit when injury reports and toxicity studies get broadcast, not buried.
Simple day-to-day behavior offers some defense. Reading labels, using gloves or goggles, and never mixing unknown substances cut risks. Old-fashioned soap and water work wonders against many messes and rarely cause harm. If you don’t know the toxicity profile of a compound, caution pays off—avoiding direct contact or inhalation can mean fewer emergency calls and, in some cases, healthier lives for everyone at home or on the job.
| Names | |
| Preferred IUPAC name | Sodium 3-(prop-2-en-1-yloxy)-2-hydroxypropane-1-sulfonate |
| Other names |
AEP Na-salt Sodium 3-(2-propen-1-yloxy)-2-hydroxypropanesulfonate Sodium 3-(allyloxy)-2-hydroxy-1-propanesulfonate 3-(Allyloxy)-2-hydroxypropanesulfonic acid sodium salt |
| Pronunciation | /ˈsəʊdiəm θriː ˈæliˌɒksi ˈtuː haɪˈdrɒksɪˌprəʊpeɪnˈsʌl.feɪt/ |
| Identifiers | |
| CAS Number | 156141-99-6 |
| Beilstein Reference | 837873 |
| ChEBI | CHEBI:136912 |
| ChEMBL | CHEMBL2103831 |
| ChemSpider | 14478465 |
| DrugBank | DB12504 |
| ECHA InfoCard | 03bcfd09-bfb8-4dba-9c72-57504c65ac82 |
| EC Number | EC 401-060-7 |
| Gmelin Reference | 98198 |
| KEGG | C14385 |
| MeSH | Sodium 3-(Allyloxy)-2-Hydroxypropanesulphonate" does not have a specific MeSH (Medical Subject Headings) term assigned. |
| PubChem CID | 10497219 |
| RTECS number | VA2148400 |
| UNII | F3F47GQ5Y9 |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID3046997 |
| Properties | |
| Chemical formula | C6H11NaO5S |
| Molar mass | 242.25 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.22 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.9 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 6.73 |
| Basicity (pKb) | 7.5 |
| Magnetic susceptibility (χ) | -44.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.459 |
| Viscosity | Viscous liquid |
| Dipole moment | 4.6286 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 276.3 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313, P302+P352 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 125 °C |
| Lethal dose or concentration | LD50 oral rat >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 20% |
| Related compounds | |
| Related compounds |
2-Hydroxy-3-prop-2-enoxypropane-1-sulfonic acid Sodium 3-(allyloxy)-2-hydroxypropane-1-sulfonate Allyl glyceryl sulfonate Glycerol monoallyl ether sulfonate sodium salt 2-Hydroxy-3-(allyloxy)propylsulfonic acid sodium salt |