Scientific work on sugar derivatives opened many doors over the past century. Advances in carbohydrate chemistry made it possible for researchers to dive deeper into the ways compounds like Sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate connect with everyday applications. Early studies in the 1960s aimed to understand salt forms of sugar acids, looking at both their reactivity and safety. The drive to understand such molecules grew with demand for safe sugar alternatives and compounds used in both medical and food spaces. Across labs in Europe, North America, and East Asia, teams sought more stable, predictable sugar salts. Over time, progress in crystallization methods and analytical testing refined these substances, making large-scale production possible and reliable for modern use.
Sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate lands at the intersection of utility and science. Described by many as a sodium salt of a carbohydrate-based sulfamate, its structure grants both reactivity and water solubility. Chemists and product designers show interest in this molecule because of its balance of low toxicity and physical stability under normal storage. While not yet a household staple, it draws industry attention both in its pure form and as part of broader formulation strategies.
Room temperature leaves Sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate as a white, crystalline solid. In most labs, researchers plunged this compound in water and found it dissolves readily. This water affinity allows easy blending in experimental and finished products. Tests report a melting point above 180°C, so standard warehouse conditions do not risk product breakdown. Researchers handling the compound confirm it carries a neutral to slightly basic pH in a 1% aqueous solution, aligning well with a variety of functional settings.
Accurate labeling builds trust—every vial of this sodium sulfamate comes emblazoned with purity level, typically above 98%, batch number, and the systematic chemical name. Manufacturers provide molecular weight, CAS registry number, recommended storage conditions (cool, dry space, sealed container), and shelf life estimations, which usually run at least two years. Safety symbols for skin and eye irritation show up alongside hazard codes where local law demands them, and clear lot traceability helps backtrack any issues during audits.
Producing this compound starts with a carefully measured carbohydrate backbone, often sourced from D-gluconic acid, which enters a reaction vessel with a sulfamating agent—most often sulfamic acid—in the presence of sodium hydroxide. The process takes place under controlled temperatures, keeping things below 60°C, to avoid charring or by-product formation. Chemists maintain vigorous stirring to speed up the reaction, carefully watch pH levels, and finish with purification, usually including crystallization and filtration. The route keeps byproduct waste low, matching modern sustainability aims.
Lab groups harness the flexible nature of its sugar core for chemical modifications. Attaching functional groups at different positions lets researchers tailor solubility and reactivity for unique applications. Some teams investigate oxidation to tweak electronic features, or reduction for targeted drug delivery research. The sulfamate group stands out as a handle for further sulfonation or cross-linking, driving both material science and pharmaceutical projects. Each additional twist in the method brings a change in physical profile, solubility, or biological reactivity.
Global collaboration brought many names for this same material. Some catalogs call it sodium glucosylsulfamate, matching the sugar source; others refer to sodium hexanoyl sulfamate or sodium gluconosulfamate. Regulatory filings sometimes stick to the long systematic title. No matter the trade name used in Europe, Japan, or the United States, core chemical structure keeps applications comparable worldwide.
Health agencies in all major markets require full safety documentation. Material safety data sheets detail recommended handling—face shield and gloves for laboratory work, plenty of ventilation, and quick access to fresh water for spills. Ingestion studies and dermal irritation reports indicate low acute toxicity, yet standard chemical hygiene protocols still apply. Bulk users and transporters follow United Nations transport codes where necessary. Procedures for storage include keeping the powder away from oxidizers and acids; strong sunlight also gets avoided to keep degradation at bay. Good laboratory practice (GLP) audits often include these details, part of a growing trust in reagent supply chains.
Interest in sodium sulfamate derivatives extends from food processing and sweetener research into realms like drug formulation and personal care. The quest for new, effective enzyme inhibitors put this molecule under the microscope in diabetes and obesity research. Cosmeceutical firms find value in its mildness and compatibility with a wide pH range, leading to trials in oral care and dermatology prototypes. Water treatment groups evaluate sulfamated carbohydrates for scaling reduction without harmful byproducts. Every sector wants reliability, so this compound’s strong physical profile turns heads.
Academic and corporate investigators keep publishing new papers each year. Recent highlights include structure-activity relationship studies, testing not just how the sodium salt behaves, but also analogues with different alkali metals or carbohydrate backbones. Funding for green chemistry approaches continues to rise, with teams searching for lower-impact ways to introduce the sulfamate group. Analytical chemists showcase advances in high-performance liquid chromatography (HPLC) and mass spectrometry for tracking purity, stability, and degradation products—even at the parts-per-billion level. Each published breakthrough reinforces the compound’s position in the innovation pipeline.
Animal testing set the foundation for safe human use. Repeated dosing studies point to low systemic toxicity at reasonable exposure levels. Oral and dermal exposure data demonstrate low irritation risk, although high purity remains a must to avoid unexpected contaminants. The compound does not bioaccumulate, lowering environmental risk. Chronic effects draw ongoing study, particularly whenever modified derivatives are introduced into consumer paths. Researchers compare outcomes side-by-side with related sodium and potassium sulfamates to identify safer or more efficient options. Cross-discipline safety reviews keep industry standards tight and public acceptance growing.
Demand for safer, gentler sugar-derived compounds rises every year. Food scientists see a future with expanded GRAS (Generally Recognized As Safe) clearances as toxicology data becomes more robust. Pharmaceutical research continues to explore extended-release and targeted delivery models using sodium sulfamate carriers. Environmental technologies count on non-toxic, biodegradable additives, driving more plant-based production routes and recycling programs for raw chemical streams. Improved scale-up technologies, automation, and digital process control offer lower costs and higher quality. As industries keep sharing data and investing in open research, sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate is set to become a model for safe, adaptable, and sustainable specialty chemicals.
Everyone knows white sugar gets most of the spotlight on ingredient labels, but for a lot of people with diabetes or weight challenges, sugar isn’t safe or practical. Sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate goes by a less intimidating name in the real world: sodium glucosamine sulfamate, though most folks recognize it better as a building block in low-calorie sweeteners, particularly acesulfame potassium. The food industry uses this compound because it lets people satisfy a sweet tooth without spiking blood sugar or adding extra calories.
Consider the everyday diet soft drink or that spoonful of sugar substitute in your morning coffee. Formulating these products would be tougher without alternatives like this. Researchers discovered only a sliver of the general population could actually taste this compound on its own—its real value comes from how it combines with other sweeteners such as aspartame. Blending it into a mix helps give a more sugar-like taste, masking aftertastes that sometimes turn folks off from sugar substitutes.
Before a molecule like this ever shows up on grocery shelves, food scientists rely on data to confirm safety. Regulatory agencies in Europe, North America, and Asia thoroughly review evidence from toxicology and long-term consumption studies. As a rule, sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate passes safety checks when used as intended, based on daily intake guidelines.
The food manufacturing process also faces strict guidelines. Quality control checks require precise amounts and routine monitoring. I’ve seen first-hand how even reputable companies dedicate entire labs to check sweetener consistency before shipping out a single case of diet candy or sugar-free gum. Lapses in this system could turn trust upside down, and industry players rarely take chances.
Doctors and pharmacists know that not all patients can handle standard sugar pills and syrups. Diabetics and people with metabolic issues rely on alternatives that don’t wreak havoc on blood sugar. Some oral medications use excipients and coatings based on sweetener chemistries related to sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate. I worked in a pharmacy where the difference between medicine someone could safely take, versus one triggering unwanted effects, sometimes came down to the filler or sweetener.
Dietary supplements and chewable vitamins also borrow this molecule’s sweetening properties. Since not every child or adult wants the aftertaste of plain ascorbic acid, these alternatives help invent products people actually stick with, convincing parents and caregivers that “medicine” doesn’t have to taste like punishment.
Any ingredient with a complex name brings hesitation. Some worry about possible health risks because stories circulate on the internet. In reality, regulators still monitor research on artificial sweeteners. Clear labeling, third-party reviews, and ongoing transparency help keep concerns down and consumer trust up. Honest public discussion—especially with input from registered dietitians and food scientists—lets consumers understand safety straight from experts, not just viral rumors.
There won’t be a one-size-fits-all solution for sweet foods and medicines. Some people want to avoid sugar entirely; others value natural sources. Both the food industry and healthcare field lean on innovative chemistry to give meaningful choices to everyone. Sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate represents more than just a line on an ingredient list—it stands as a symbol of progress balancing safety, enjoyment, and health goals in daily life.
The long chemical name, Sodium (2R,3R,4S,5R)-3,4,5,6-Tetrahydroxy-1-Oxohexan-2-Yl)Sulfamate, sounds more like an equation than something found in a kitchen. This compound doesn’t pop up in daily chatter, but it’s closely related to the world of food additives and artificial sweeteners. It’s a derivative found in several sugar substitutes, products that sit on supermarket shelves promising fewer calories and a sweet taste without spikes in blood sugar.
Curiosity pulls many people to research what they put in their bodies, especially with new or unfamiliar names. Regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) oversee the approval of such compounds. Their scientists conduct both short-term and long-term studies, looking for signs of toxicity, carcinogenicity, and potential dietary risks.
For this family of compounds, agencies have focused on their breakdown in the body, possible allergic reactions, and metabolic pathways. They have paid particular attention to sulfamates because similar molecules have caused issues in high doses in some animal studies—such as kidney stress and potential nervous system impacts. Not every result from a rat cage translates to humans. Yet, scientists can’t ignore red flags.
Misinformation swirls quickly online. I’ve seen big headlines exaggerated by blogs or influencers without science backgrounds. Honest assessment starts with reviewed studies. In the case of sodium (2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate, published research shows that in reasonable amounts—those similar to what a person would find in an ordinary serving—no significant harm has surfaced for healthy adults. Known brands using related sulfamates have been approved by multiple agencies, and product recalls linked to this precise structure don’t crop up in public records.
Not everyone processes chemicals the same. People with kidney problems, those on certain medications, or who are sensitive to sulfamates face a different risk landscape. Doctors and nutritionists keep an eye on developments, ready to spot patterns of side effects or rare reactions. If a compound caused mass problems, regulatory agencies move quickly, as seen with multiple artificial sweeteners pulled from shelves decades ago.
For many, non-nutritive sweeteners offer an alternative to sugar, supporting weight control and blood sugar management. Introducing a new sweetener or additive often triggers scrutiny and public debate, but regular check-ups by both agencies and independent labs continue even after products go to market.
Open conversations with health professionals help the most. People living with diabetes or chronic illnesses often rely on several types of sweeteners. Trust builds through transparency and access to solid science, with published studies forming the backbone of most dietary guidelines. Harvesting that experience means more than reading a label or a single sensational article—dig for the studies, ask tough questions, and look past the packaging.
Food safety isn’t a one-and-done test. Scientists and regulators adapt as new data roll in. Continued funding for independent research, quicker reporting of side effects, and honest labeling practices push the industry in the right direction. Encouraging researchers to share negative findings along with positive keeps the full picture in view—not just the shiny results.
People vote with their forks and their wallets. Reading up, asking questions, and pushing companies for more information leads to smarter choices. If a product raises concerns, other options always exist. Staying informed is the surest path to safe decisions at the table.
There’s nothing more frustrating than running an experiment and finding your reagent clumped up or degraded. Years in research labs taught me one lesson fast: how you store a compound can make or break your work. With sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate, proper storage isn’t just a good suggestion; it’s non-negotiable for keeping this chemical stable and safe.
A big part of a chemist’s day revolves around careful housekeeping. Humidity, light, and temperature all play big roles in how a sensitive sugar-sulfamate behaves. It’s easy to underestimate just how badly a few degrees or an open container cap can spoil a batch, but one ruined sample turns belief into muscle memory.
This compound draws water from the air far faster than you might expect. Hydrophilic groups in its structure act like a magnet for humidity. Left uncapped on the bench, it soaks up moisture, dissolving into a sticky mess. That kind of contamination cuts shelf life, clogs pipettes, and throws off weighing. Instead of storing it loosely, keep the powder sealed tight inside a desiccator or tightly capped vial. Investing in a desiccant bag always pays off.
Air also brings another threat: oxygen. Some sugar derivatives oxidize with shameful ease. Keeping the reagent flushed with an inert gas—like argon or nitrogen—means less chance of unwanted side reactions. Even for short-term storage, every chemist I know keeps a reliable gas line handy for sensitive organics.
Room temperature might seem harmless, but elevated temperatures accelerate all sorts of chemical breakdown. If you’ve spent time cataloguing samples, you’ve probably seen containers buckle on a hot day. Most labs use cold storage for a reason. A temperature between 2°C and 8°C (found in any standard laboratory fridge) offers a safe range for sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate. Colder storage slows down decomposition and gives you peace of mind—especially for expensive or rare reagents.
On a personal note, I’ve even found dry ice packs to help during transport if moving compounds between labs or buildings. Leaving temperature-sensitive material in a warm corridor or delivery crate proves costly—chemicals degrade before even making it onto the shelf.
No researcher wants to discover their material doesn’t work due to careless handling. Using separate spatulas or tools for each reagent prevents cross-contact. Even a grain of dust or a drop of moisture can introduce impurities, complicate purification, or ruin results. I keep a set of utensils for sensitive sugars and make sure everything’s bone-dry before dipping them into a fresh vial.
Experience shows, a clear label with the compound name, batch number, and date ensures everyone knows how old each reagent is. Leaving out these details leads to confusion down the road. Accurate records help catch expiry, prevent accidental mix-ups, and support full traceability in publications and audits.
For multi-year projects, aliquoting into smaller vials guards against repeated exposure to air and moisture. Each time you open the main supply, you risk contamination. Breaking into single-use portions reduces this threat. I’ve never lost a batch stored this way, and it saves plenty of headaches during follow-up experiments.
Reliable storage practices cut down waste and prevent dangerous surprises. In research, every little precaution can save hours of troubleshooting and protect both results and budgets.
It’s easy to overlook what goes inside the stuff we use every day. With complex names like Sodium ((2R,3R,4S,5R)-3,4,5,6-Tetrahydroxy-1-Oxohexan-2-Yl)Sulfamate, most folks just want to know: is it safe? Are the side effects worth worrying about? Cracking open a medical or chemical database won't always give a straight answer. This isn’t a common compound you hear about at the pharmacy or even in big scientific news. Right now, information on direct side effects in humans demands a deeper dig.
Looking at relatives of sulfamate-based chemicals helps shed some light. For example, drugs like topiramate and zonisamide share a sulfamate group and bring certain risks like fatigue, nausea, kidney stones, and rare skin reactions. These medicines go through rigorous testing. None of that guarantees every sulfamate will react the same way, but it does raise red flags.
With sugars in the mix, as the “tetrahydroxy-1-oxohexan” part hints, there’s a worry about altered blood sugar levels for some. People dealing with diabetes need to check if anything on their plate (or in their medicine cabinet) messes with their numbers. Mix sulfamates with that, and it signals the need for more research on metabolism and possible allergic reactions.
In animal trials, sulfamate-related compounds sometimes lead to issues with liver enzymes or blood cell counts. The health risks from long-term exposure—whether through food, drink, or medication—never fade away easily. While animal data isn’t a perfect match for people, it offers hints. Stomach discomfort, changes in the way the body breaks down other chemicals, and even liver stress pop up more often in these studies.
A rise in research on food additives and artificial sweeteners shows, too, that certain sulfamates have caused headaches or gut irritation, especially in folks already sensitive to new ingredients. The issue gets sticky because these compounds sometimes build up in the body, leaving doctors guessing about the tipping point for trouble.
Few things create concern like the unknown, and uncharted chemicals haven’t earned the benefit of the doubt. People trust science most when real-world testing lines up with clear results. Until safety trials wrap up, and experts publish peer-reviewed findings, rolling out something as mysterious as Sodium ((2R,3R,4S,5R)-3,4,5,6-Tetrahydroxy-1-Oxohexan-2-Yl)Sulfamate isn’t worth the gamble, especially when there are safer, time-tested options for most needs.
The best fix starts with transparency. Ingredient panels need to spell out what’s inside and share what we know about risks. Researchers, companies, and regulators all play a part, from running animal and volunteer studies to sharing side effects as they show up. Doctors and consumers both deserve open access to evidence and a place to raise questions when something new lands on the market.
Not every chemical with a shiny name belongs in a daily routine. Trust in a product grows out of facts, not hope. If questions linger, call the company making the compound, check for published science and review opinions from trusted watchdog groups. Waiting until researchers nail down the truth keeps random surprises off your plate and out of your home.
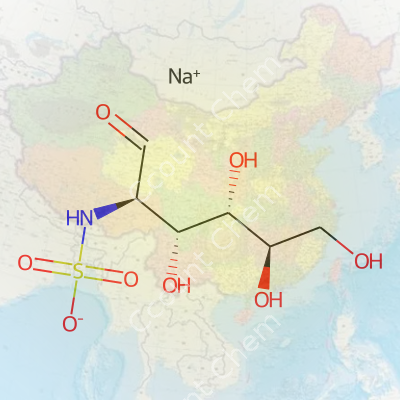
The name Sodium ((2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl)sulfamate catches the eye, not only for its length, but for its clear nod to specific molecular twists and turns. Those R’s and S’s in the formula point to how carbon atoms arrange their hands on the molecular steering wheel, creating right- or left-handed versions. Reading this, organic chemists spot a hexose backbone (six carbons), loaded with hydroxy groups for water bonding, and a sulfamate group for extra functionality. Think of a sugar, like glucose, that's been customized for a brand new job.
This molecule comes out with a formula: C6H11NO8SNa. There’s a sodium salt, making it soluble. The backbone is derived from a hexose, suggesting deep roots in carbohydrate chemistry — a field that has powered biomedicine and food science. The sulfamate group gets chemists’ attention because of its reactivity and role in medicinal chemistry.
Sodium-based sugars often show up asking for new jobs in the pharmaceutical world. A classic example is how the sulfamate group can help molecules work as enzyme inhibitors. Take the antiepileptic drug topiramate: slight changes to a sugar molecule’s structure, especially sticking on a sulfamate, turned it into a potent medicine. In small-scale research, teams experiment with molecules like this for diabetes and rare disorders. There's reason to keep watch on these tweaks.
Nature cares a lot about the direction a chemist twists a molecule. Human enzymes, receptors, and even taste buds notice the difference when a molecule bends rightward or leftward. In clinics and labs, one twist can help, another can harm or do nothing. Building molecules with precise chirality costs more upfront, but clinical safety depends on it. Sticking to the right layout (like the (2R,3R,4S,5R) pattern above) makes a difference in real experiments—I've seen projects falter when the wrong chirality slipped into a drug batch.
Carbohydrate sulfamates aren’t just chemical curiosities. They step into enzyme research, help target infections, and, in emerging studies, show cancer-fighting promise. Sodium as a counterion boosts water solubility, a crucial point for any compound aiming for an injectable form or a pill that dissolves in the gut.
Sustainable chemistry also roots for these molecules. Modern routes try to build them from plant sugars, skipping over harsh solvents or heavy metals. A few years ago, working alongside researchers in green synthesis, I saw how these sugars could be transformed safely, benefiting both factory workers and the environment.
Tighter regulations and better scientific practice demand certainty about any synthetic compound’s structure. That means clear analytical data for every batch — chromatography, NMR, infra-red tests — and triple-checking the chirality. Whole facilities now stake their reputation on supplying exact structures, like this sodium sulfamate, to the pharmaceutical world.
With rare diseases and metabolic disorders driving new drug research, sodium hexose sulfamates wait in the wings. More university teams now use 3D modeling, green chemistry, and precision analytics to build out this class for specific targets. Public health and industry stand to benefit when chemists share insights, control impurities, and stick closely to nature’s blueprints when aiming for safer, better results.
| Names | |
| Preferred IUPAC name | Sodium (2R,3R,4S,5R)-3,4,5,6-tetrahydroxy-1-oxohexan-2-yl sulfamate |
| Other names |
D-Glucosamine-6-sulfamate sodium salt GlcN6SNa |
| Pronunciation | /ˈsəʊdiəm ˌtɛtrəˈhaɪdrɒksi ˈɒksəʊˌhɛkˌsæn tuː ˈsʌlfəˌmeɪt/ |
| Identifiers | |
| CAS Number | 138-44-9 |
| 3D model (JSmol) | `/data/3d/6/8b6/SODIUM_((2R,3R,4S,5R)-3,4,5,6-TETRAHYDROXY-1-OXOHEXAN-2-YL)SULFAMATE_1936.jmol` |
| Beilstein Reference | 1732421 |
| ChEBI | CHEBI:9547 |
| ChEMBL | CHEMBL2103838 |
| ChemSpider | 21542036 |
| DrugBank | DB00331 |
| ECHA InfoCard | 05b3bb3d-d0e2-4a3c-a038-b99b2d8bc3f8 |
| EC Number | EC 3.2.1.22 |
| Gmelin Reference | 104188 |
| KEGG | C19678 |
| MeSH | D-glucitol 6-sulfamate |
| PubChem CID | 10427621 |
| RTECS number | WH6650000 |
| UNII | INT1L6S7N9 |
| UN number | 2811 |
| CompTox Dashboard (EPA) | DTXSID20988398 |
| Properties | |
| Chemical formula | C6H12NO8SNa |
| Molar mass | 257.201 g/mol |
| Appearance | white solid |
| Odor | Odorless |
| Density | 1.87 g/cm³ |
| Solubility in water | soluble |
| log P | -3.6 |
| Acidity (pKa) | pKa = 7.2 |
| Basicity (pKb) | 1.99 |
| Magnetic susceptibility (χ) | -85.0 · 10^-6 cm³/mol |
| Refractive index (nD) | 1.528 |
| Viscosity | 23 cP (20 °C) |
| Dipole moment | 7.0611 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 247.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1613.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2105.9 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A10BD07 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 16,000 mg/kg |
| NIOSH | WNQWMRGSPRZRAZ-UHFFFAOYSA-M |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 0.003 mg/kg bw |
| Related compounds | |
| Related compounds |
Acesulfame potassium Saccharin Sucralose Cyclamate Dulcin |