Sodium 2-hydroxyethanesulphonate traces its roots back to the golden age of chemical innovation, right alongside booming industrial development in the twentieth century. Chemists first explored sulfonic acid derivatives when detergent manufacturing demanded alternatives to harsher phosphates. This compound, also known to many as sodium isethionate, offered something different: it presented a milder sulfonate group that didn’t strip or corrode everything in its path. The move sparked fresh ideas for cleaning agents, personal care products, and textile processes. People in labs and factories saw sodium 2-hydroxyethanesulphonate as a friendlier ingredient that reduced environmental impact at a time when pollution began catching the world's attention. My work in industrial R&D circles reminds me how much real progress often hinges on tweaks to established molecules and a careful eye on the world’s changing demands.
Sodium 2-hydroxyethanesulphonate comes as a white, odorless powder or sometimes as small granules. It serves both as a key raw material and as a co-surfactant, thanks to its ability to dissolve easily in water. Typical buyers include detergent manufacturers, cosmetics formulators, and laboratories seeking mild yet functional chemical building blocks. The chemical’s market names run the spectrum—sodium isethionate, 2-hydroxyethanesulphonic acid sodium salt, and simply isethionate sodium salt. Clients in the personal care market always look for ingredients that handle both form and function, and this one ticks those boxes pretty soundly.
This salt boasts a melting point near 250°C (decomposes) and does not ignite easily, which makes handling and storage less complicated. Water-solubility remains its most valued physical trait; a property that opens up countless formulation options. The chemical structure fields a sulfonate group paired with a hydroxyethyl chain, lending itself well to reactions for surfactant and detergent work. It doesn’t carry a strong odor or color, so it fits neatly into a range of products that demand cleanliness and neutrality. The pH level of its aqueous solutions typically lands around 6–8, aligning it with other mild surfactants used in personal care and specialty cleaners. My years in formulation chemistry showed me that such traits mean fewer side reactions or unexpected surprises, a practical win for production lines.
Producers supply sodium 2-hydroxyethanesulphonate in bags, drums, or bulk containers, listing country of origin, batch number, gross and net weight, and purity level—usually 98 percent or better by dry weight. Certificates of analysis check for chloride, sulfate, and heavy metal contaminants, with each batch expected to fall well below industry acceptance limits. Hazard labels advise protective gloves and eye protection due to drying and mild irritancy risks. There’s growing interest in clear provenance and sustainability marking, so some suppliers offer detailed traceability info on leaflets or integrated QR codes right on the packaging. Labs appreciate this transparency, since many research protocols require detailed sourcing histories for repeatability and regulatory compliance.
Industrial-scale production of sodium 2-hydroxyethanesulphonate usually kicks off with ethylene oxide and sodium bisulfite in aqueous solution. Direct addition at controlled temperature makes for a tidy, safe, and scalable route. Technicians rely on constant stirring and careful control of reactant feed, since ethylene oxide brings both volatility and toxicity to the table. Automated dosing keeps exposure low, minimizing both waste and safety incidents. Post-reaction stages involve filtration and drying to get the compound to a stable, powdery state. Lower grade preparations may leave trace inorganic salts, but high-purity demand calls for careful washing and sometimes vacuum drying. Shops that don’t cut corners save money on rework and distribution headaches, an experience echoed by many long-timers in process control roles.
Sodium 2-hydroxyethanesulphonate finds its way into reactions aiming for gentle sulfonate transfers or where ready water solubility is vital. It resists hydrolysis under normal storage and mixing conditions. When used as a building block, its hydroxy group can react with fatty acid chlorides to develop mild surfactants—one reason sodium cocoyl isethionate ended up so popular in sulfate-free hair and body bars. In lab environments, it serves as a starting point for synthesizing zwitterionic surfactants and for studies probing sulfonate reactivity. Reactions with strong oxidizers need careful management, but the vast majority of industrial applications see this molecule paired with fatty acids, glycols, or alcohols under neutral or slightly alkaline conditions.
Common industry names include sodium isethionate, sodium 2-hydroxyethanesulfonate, sodium oxyethanesulfonate, and the snappier SIES. Each term turns up in buyer guides, chemical supply catalogs, and trade documents. Regulatory certificates sometimes split hairs over nomenclature, so technical teams use CAS numbers or EC identifiers for clarity. In my procurement experience, using the most precise name avoids costly mix-ups—and saves many late-night calls to suppliers fixing preventable problems.
Handling sodium 2-hydroxyethanesulphonate safely means respecting its mild irritant profile and controlling airborne dust. Eye protection and gloves come standard on shop floors, and good ventilation helps. Training materials stress prompt cleanup of spills using dry absorbent, not water jets, so as to avoid slip hazards. Material safety data sheets spell out key risks and set workplace exposure limits, which remain comfortably high due to the compound’s low systemic toxicity. Waste disposal typically involves dilution and neutralization before discharging into municipal wastewater, though regions with stricter rules may demand more advanced treatments. Lawyers and insurance agents keep a close eye on REACH and EPA assessments, knowing that shifting classification can upend compliance programs overnight.
Markets for sodium 2-hydroxyethanesulphonate revolve around personal care (shampoos, skin cleansers, bath bars), detergents, textile auxiliaries, and sometimes photography. The appeal starts with skincare and haircare, since it performs as a gentle surfactant and offers desirable foaming without the drying bite of sulfates. Bar soaps packed with sodium cocoyl isethionate owe their silky lather and gentle touch to this core ingredient. Laundry and hard surface cleaners also lean on its water solubility and environmental compatibility. Textile manufacturers use it during wet processing of fibers, noting improved dye leveling and fewer deposit problems. Folks chasing “softer chemistry” like its low skin irritation and ready biodegradability—features that draw in regulation-conscious brands.
Current research around sodium 2-hydroxyethanesulphonate focuses on greener production routes, such as using lower-temperature catalysts or bio-derived ethylene oxide alternatives. R&D teams push for surfactant blends that match or beat classic sulphated products in foaming, mildness, and rinseability without carrying the baggage of persistent organic pollution. In my own work with surfactant panels, newer blends based on isethionate derivatives show less skin barrier disruption and greater tolerance by sensitive skin types. Universities and industry consortia dig into its sulfonate chemistry, seeking to unlock more biodegradable polymers and improve solubilization for drugs or specialty actives. Many labs publish head-to-head testing that gives real-world metrics—foam breakdown, irritancy scores, and ultimate wastewater trace levels.
Acute toxicity tests rank sodium 2-hydroxyethanesulphonate as safe for commercial applications when used properly. Skin irritation remains mild, and inhalation risks fall low if dust controls are followed. Animal studies point to high LD50 threshold values; major regulators, including the US and EU bodies, regard it as a low-concern chemical for both consumer and environmental health. Disposal into municipal water streams rarely threatens aquatic life given its ready breakdown. Airlines and manufacturers still flag it as non-food grade, keeping careful buffers between chemical and ingestible product chains. Testing continues on long-term exposure and on derivatives used in even more sensitive applications, but the weight of evidence stacks on the side of minimal toxicity and high user safety.
Growing demand for mild, sustainable cleaning and skincare options will keep pushing sodium 2-hydroxyethanesulphonate and its derivatives into broader applications. Fragrance-free and sulfate-free product trends show no sign of slowing, and this ingredient wins support for balancing gentle touch with strong cleansing action. Environmental pressures are mounting to replace persistent, high-risk surfactants; sodium 2-hydroxyethanesulphonate stands out as a proven, reliable substitute. Manufacturing advances in greener chemistry and more resource-efficient processing promise to trim costs and carbon footprints further. As more companies publish full ingredient disclosure, those relying on this compound gain a marketing edge with better transparency. I’ve seen brand trust rise when people recognize and understand labels, and the low-cost, low-toxicity record of this ingredient puts it ahead in both public and regulatory eyes.
You come across big chemical names like sodium 2-hydroxyethanesulphonate and probably think only scientists care. I used to glance over these ingredients on product packaging, too, but the more you dig, the more you realize how many common products depend on such compounds. Sodium 2-hydroxyethanesulphonate, often called isethionate, shows up in places we don’t always expect.
Step into the shower, pick up your favorite cleansing bar or shampoo, check the label, and you will see some version of sodium isethionate hanging around. Companies use it to help create the creamy, easy-to-rinse foam in syndet bars—those are soap substitutes that clean without stripping the skin. My experience with dry skin made me reach for these ‘gentle’ cleansers, and they worked noticeably better than regular soap. The reason? This molecule acts as a surfactant. Surfactants help water and oil blend so you can wash away dirt without harshness. Sodium 2-hydroxyethanesulphonate offers this property while causing less irritation compared to classic soap ingredients.
If you have sensitive skin or a baby at home, you might have used a product that owes its mildness to this ingredient. Brands market ‘no-tears’ baby shampoos using isethionate because of the way it balances cleaning power and gentleness.
The uses don’t end at the bathroom shelf. Textile processing grabs this material for use as a dyeing assistant. It works in water treatment too, helping chemicals dissolve into solution better. In my college chemistry days, we studied water softeners and how certain compounds kept pipes from scaling up or fouling. Turns out, sodium 2-hydroxyethanesulphonate can help control water properties in large systems like laundries or manufacturing lines, cutting maintenance costs and improving output quality.
Some liquid detergents also gain stability and solubility from it. Because it dissolves well and mixes easily, it lets products deliver their cleaning punch at even low temperatures. For laundry in colder countries, or anyone who washes with cool water, this keeps whites brighter and removes more stains. It helps prevent those annoying soap scum deposits on clothes and machines as well.
A big story around chemicals today focuses on eco-friendliness. Sodium 2-hydroxyethanesulphonate is known for low toxicity and breaks down in the environment better than some older surfactants. This makes it appealing for those of us watching out for water pollution or sensitive aquatic life. It’s not perfect—no industrial chemical is—but using less persistent cleaning agents marks a step toward a healthier environment.
Challenges do crop up. Supply chain fluctuations or price hikes in the raw materials needed to make isethionate can filter through to consumer prices. As more brands lean into ‘gentle’ and ‘green’ labels, demand grows, and so do cost pressures.
A practical fix would be more local production, less reliance on complex international sourcing, and ongoing work to create bio-based alternatives. Research continues to tweak molecule structure for even less skin reactivity and faster disposal in wastewater plants. From what I’ve seen, collaboration between chemical makers and public health experts pays off here—real testing and honest labeling help consumers pick safer products.
Sodium 2-hydroxyethanesulphonate doesn’t get splashy headlines, but its role shows up whenever someone reaches for mild soap, cleans water, or tries to ease their laundry routine. Watching how it threads through daily life brings chemistry down to earth, connecting the lab bench with comfort in the home.
Sodium 2-Hydroxyethanesulphonate, sometimes listed as sodium isethionate, pops up in a lot of personal care products. You’ll spot it most in soaps, shampoos, and some cleansers. The chemical works as a surfactant, helping water and oil mix so dirt and oils can wash away. On ingredient labels, this one often sounds intimidating, but its popularity keeps growing. Many products marketed as ‘gentle’ or ‘for sensitive skin’ rely on it.
The question keeps coming up: Is it safe for people? Regulatory agencies like the FDA and the European Chemicals Agency keep records on chemicals and ingredients in consumer items. So do independent safety groups. Sodium isethionate already has a track record in soaps for decades. Several expert panels, including the Cosmetic Ingredient Review (CIR), checked its safety profile in both rinse-off and leave-on formulas.
In my years dealing with common skin issues, irritants in personal products turn out to be a big culprit. The CIR has not found sodium isethionate to cause allergies or skin sensitization at the concentrations used in cosmetics. The European Union allows its use in personal care and toiletries, without putting strong restrictions in place. Most reported reactions show up as mild and rare, especially compared to harsher cleansers like sulfates.
What stands out is the amount of toxicity data available. Studies on humans and animals both look for immediate dangers or long-term health effects. According to research, sodium 2-hydroxyethanesulphonate does not absorb deeply into the skin. Once applied, most of it rinses away with water. Ingestion studies, which matter for accidental exposure, show the body can break it down and flush it out. No build-up in organs. That’s a solid sign.
Groups monitoring the environment check if washing these products down the drain harms wildlife or water. Current data doesn’t point to big risks in environmental toxicity, especially since wastewater plants remove most sulfonic acids from water.
Even for substances with long, easy records, no product sits at absolute zero risk. A handful of people may have sensitive skin or other allergies. Adding a new cleanser or shampoo? Testing a small area first never hurts, especially if you’ve reacted badly to soaps before. Kids’ skin handles chemicals differently than adults’—extra caution helps there.
Another concern comes from the industry overall, not just this one ingredient. Some products load up on a mix of mild and harsh chemicals. Sometimes, gentler choices get overshadowed by fragrances, alcohols, or preservatives that actually cause more trouble. I always suggest reading the whole ingredient list, not just searching for a single word.
Looking for reputable brands with transparency helps. Companies that publish safety test results and respond to customer feedback offer better peace of mind. Dermatologists and family doctors keep up with the latest findings—ask about your specific case, especially for babies and people with chronic skin problems. For manufacturers, batch testing and traceability cut down risks from contamination or unexpected impurities.
Folks worried about unknowns can turn to databases like EWG’s Skin Deep or check regulatory decisions in the U.S., European Union, and Japan. This gives some control back to consumers, which matters in a market flooded with claims and buzzwords.
Managing chemicals in a lab or warehouse doesn’t just call for skill—it asks for respect. Sodium 2-Hydroxyethanesulphonate, better known in some circles as isethionate, sees use in cleaning agents, personal care items, and a range of industrial products. Anyone working near this substance should recognize how storage and handling choices shape both product quality and worker safety.
I once saw a facility turn a routine product into a problem by ignoring temperature swings and allowing a chemical like this to sit in sunlight. That mistake spelled trouble later on. Sodium 2-Hydroxyethanesulphonate holds up best in a cool, dry spot, away from direct sunlight or moisture. Any leak from the roof or floor tells you it's time to rethink storage. Piling up bags or drums near water sources risks clumping, spoilage, and unwanted reactions with other stored materials.
Most packaging keeps out water, but relying on luck tempts fate. Dry, sealed containers extend shelf life and lower the risk that spilled coffee or a flood will spoil the batch. Plenty of companies keep it in polyethylene or high-density polyethylene bins—these cut down on chemical reactions that steel might trigger. Once, our shop stored some chemicals in a rusty steel bin. What came out months later looked nothing like the original order sheet.
Direct skin or eye contact leads to irritation. Gloves, goggles, and a dust mask carry more sense than bravado. It never pays to skip proper work clothes or the habit of hand washing after shifts. Having worked alongside teams that huffed and puffed at the idea of PPE, I watched the ones with long sleeves and masks avoid sick days and skin rashes. Simple habits save trips to the clinic.
Pour this chemical gently to kick up less dust. A clean, ventilated spot helps limit what hangs in the air. After a long day, I’ve often seen folks sweeping up floors and wiping benches. Housekeeping works faster in teams. Air vents or fume hoods control dust, and they matter all year, not just in allergy season.
This chemical itself won’t start a fire, but it won’t help put one out either. Keep it away from strong oxidizers and acids—they don’t mix well and make a bad day worse. I learned early to check labels twice before opening any drum near a bottle of bleach or nitric acid.
The right habit is to label every bottle and container right after filling. No one likes mystery powders in dusty bags a year later. Clear labeling builds trust among coworkers—nobody wants a guesswork guessing game in their storage space.
Safety talks and posted reminders sometimes fade into wallpaper. Regular walkthroughs catch moldy corners, leaky drums, or boxes stacked too high. Every new hire should step through real-world lessons—watching, not just reading, what safe handling looks like. Tracking spills in a logbook and rewarding careful workers keeps routines sharp and habits honest. In dozens of labs I’ve visited, the cleanest stores stay that way thanks to stubborn pride, not luck.
Forging good habits means listening to folks who fill, lift, dump, and seal up chemicals year-round. Their practical tweaks carry more weight than any policy binder. Safety sticks when everyone speaks up and the small wins—like spotless shelves and zero near-misses—get recognized by the team.
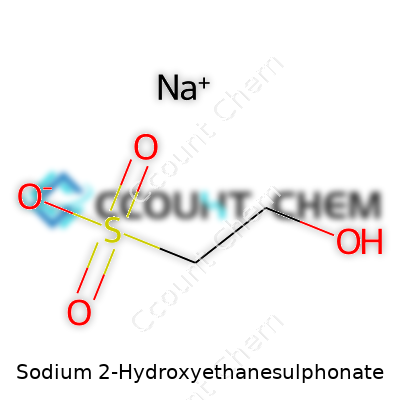
Chemicals like Sodium 2-Hydroxyethanesulphonate can sound intimidating, but the role they play is fundamental. At its heart, this molecule consists of sodium (Na), two-carbon chain with a hydroxyl group (-OH) and a sulfonate group (-SO3). Through my experience working on product labels and research articles, people tend to glaze over the technicalities, but understanding what’s in a compound is half the battle to using it responsibly. Its chemical formula: C2H5NaO4S. That combo lines up with the systematic name too—sodium sits with 2-hydroxyethanesulphonate, pretty straightforward if you look beneath the surface.
So, what’s really there? Two carbons hook up in a chain, one carrying a hydroxyl (-OH) and the other trailing a sulfonate group (-SO3). Sodium tags along by balancing the charge. Each atom has a role, setting up the molecule as more than a jumble of letters and numbers. Getting a grip on these building blocks isn’t just for chemists in white coats. Students in labs and workers dealing with industrial detergents bump into this stuff in real life. It all reflects chemistry’s place beyond textbooks—ingredients like this turn up in everything from cleaning up textiles to making sure your skin creams don’t dry out.
Misunderstanding a formula isn’t a small slip. I’ve seen folks muddle safety protocols or store compounds in the wrong spot just because the chemistry seemed too complex. Sodium 2-Hydroxyethanesulphonate’s presence in skin-friendly products comes from its structure. The sulfonate group brings water-attracting power, letting it dissolve and move smoothly in solutions. If someone only scans “sodium” and “sulfonate” on a label, they might jump to conclusions about harshness. Reality is, in diluted forms, this compound plays nice with skin and fabrics.
Mistakes can show up when information isn’t clear. Too many safety data sheets bury the essentials in jargon. From hands-on experience correcting chemical inventories, people need the raw formula, but also a breakdown—what do those atoms mean for day-to-day use? Training sessions work best with visuals, models, quick quizzes—not just technical lingo. Teams that translate formulas into real-life scenarios handle chemicals safer, spot mix-ups early, and waste less time double-checking everything.
Labeling laws, like the Globally Harmonized System (GHS), give companies a nudge toward better transparency. Still, labels alone won’t fix confusion. Community outreach, school workshops, and partner programs with local businesses can knock down barriers. Getting hands dirty in a classroom lab, or breaking down a formula on a worksite allows the info to stick. This makes a case for practical science education—connecting the dots between formulas, functions, and real impacts.
Sodium 2-Hydroxyethanesulphonate’s chemical formula—C2H5NaO4S—holds more than just academic interest. It shapes how supplies work, how products feel on our skin, and how industries adapt to safety rules. Strong chemistry education and workplace training give everyone—from students to warehouse staff—the confidence to read, use, and respect what’s in the bottle.
Sodium 2-hydroxyethanesulphonate shows up mostly as an intermediate in chemical processes, a buffer in personal care formulas, and sometimes as a stabilizer in industrial production. Manufacturers usually keep a close eye on how it handles various temperatures and moistures, but using it outside well-confirmed recipes can lead to problems. People rarely encounter this compound in day-to-day life, yet it can arrive in cleaning agents, personal hygiene items, or laboratory environments.
Direct skin or eye contact creates risk, especially in concentrated solutions. Reports show moderate irritation upon exposure, more so for lab workers handling flakes or powder. Accidental spillage rarely causes injury at low concentrations, but splashes around the mouth or nose need immediate rinsing. Everyone working with it should wear gloves, goggles, and a mask to dodge dust or droplets, just like handling any strong salt.
If swallowed in quantity, animal studies reveal gastrointestinal stress and mild toxicity, including upset stomach or a burning throat sensation. For most people, such contact remains rare unless living with an unsafe storage situation. Factory settings require strict storage policies to avoid mixing it with strong acids or oxidizers, two groups of chemicals that can turn a stable salt into a hazardous release of heat or even gas under bad conditions.
Chemists flag powerful oxidizing agents as a clear danger. Mixing sodium 2-hydroxyethanesulphonate with these substances sometimes leads to unwanted reactions—sometimes rust, sometimes a tint, sometimes a stronger hazard. It’s well known not to store it by acetyl chloride or sulfuric acid, as those acids can break apart the molecule, releasing corrosive vapors and causing rapid temperature shifts in closed containers.
Even mixing it with other sulphonates doesn’t guarantee smooth sailing: byproducts sometimes form, clouding batches or throwing off pH balances that operators rely on for safety. Handling with iron, copper, or other reactive metals often speeds up degradation or sparks a mild heat release—nothing spectacular, but enough to matter at large scales or over long timeframes. Keeping storage vessels clean and using stable plastics or glassware gives the safest route at most facilities.
Anyone transporting or mixing sodium 2-hydroxyethanesulphonate should pay attention to the Material Safety Data Sheet (MSDS) instructions. This advice isn’t just for show; spill kits, isolation of incompatible substances, and good ventilation keep accidents to a minimum. People sometimes become lax with “safer” salts versus those with bigger hazard labels, but repeated exposure—over weeks or months—can build up allergic or asthmatic reactions, especially among janitors, lab techs, or factory workers.
Rinsing spills quickly with water keeps residue from spreading, but waste management plans must address possible runoff into waterways, where this salt may stress aquatic life if concentrations spike. Regulations in Europe and the US now demand that facilities keep full logs of chemical use and waste disposal, which pushes producers to reexamine mixtures and keep substitutions for more eco-friendly compounds at the ready.
Understanding sodium 2-hydroxyethanesulphonate comes down to following the known science, enforcing clear protocols, and keeping communication open between supervisors and staff. Problems usually flare up when training gets rushed or companies cut corners with equipment and labeling. Investing in frequent retraining, regular inventory audits, and community outreach helps manufacturers show responsibility—and keeps people safer both at work and in the neighborhoods nearby.
| Names | |
| Preferred IUPAC name | Sodium 2-hydroxyethane-1-sulfonate |
| Other names |
Isethionic acid sodium salt Sodium isethionate 2-Hydroxyethanesulfonic acid sodium salt Ethanesulfonic acid, 2-hydroxy-, monosodium salt |
| Pronunciation | /ˈsoʊdiəm tuː haɪˌdrɒksiˌɛθeɪnˈsʌlfoʊneɪt/ |
| Identifiers | |
| CAS Number | 1562-00-1 |
| 3D model (JSmol) | `COCCS(=O)(=O)[O-].[Na+]` |
| Beilstein Reference | 803920 |
| ChEBI | CHEBI:9120 |
| ChEMBL | CHEMBL1352 |
| ChemSpider | 10799 |
| DrugBank | DB13953 |
| ECHA InfoCard | 100.007.724 |
| EC Number | 221-183-3 |
| Gmelin Reference | 152060 |
| KEGG | C02455 |
| MeSH | D017356 |
| PubChem CID | 23665773 |
| RTECS number | VL7875000 |
| UNII | 03P5MQ0KN1 |
| UN number | UN number: "UN2811 |
| Properties | |
| Chemical formula | C2H5NaO4S |
| Molar mass | 142.15 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.48 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -3.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.5 |
| Magnetic susceptibility (χ) | -30.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.464 |
| Viscosity | 100 mPa.s (20°C, 40% in H₂O) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 152.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1076.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -948.6 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | B05CB03 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 215°C |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 oral rat 4200 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 5000 mg/kg |
| NIOSH | WV5775000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Ethanesulfonic acid Isopropanesulfonic acid Methanesulfonic acid 2-Hydroxyethanesulfonic acid Sodium methanesulfonate |