Niche specialty chemicals like Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate sit in a unique spot where academic chemistry meets real-world manufacturing. Tracing back to the late 1970s, work from researchers in Europe explored the potential of sulfonate esters for industrial and pharmaceutical synthesis. Growing interest came from the need to improve molecular stability during complex reactions. I first ran across references to this compound in German chemistry literature, where it cropped up in reaction pathways for aromatic sulfonation. Since then, smarter synthesis pathways have cut out waste and minimized byproducts, all to serve rising demands for tailored sulfonates for everything from polymer production to advanced pharmaceuticals.
Practical uses for Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate show up in a few different fields. At its core, this molecule offers strong sulfonating capacity, supporting work as an intermediate in organic synthesis. Labs with an eye on specialty intermediates—think custom molecule builders, polymer researchers, and pharmaceutical developers—often turn to this sodium salt to carry out modifications that can’t be achieved with bulkier or less selective reagents. Reliable suppliers sell the crystalline powder form, because it stores well and can be weighed or dissolved easily.
This compound takes the form of a white to off-white crystalline material. Dry storage keeps it free from clumping, and under normal room temperature it remains stable. Its high water solubility makes it easy to introduce into aqueous solutions, but I’ve seen it used in mixed organic solvents too, usually where fine-tuned reactivity matters. Melting points usually land in the 190–210°C range. The structure contains both a carboxylic acid ester (from the benzoyl group) and a sulfonate group, meaning the molecule has a split personality—with hydrophilic and hydrophobic portions creating useful properties for chemical design. Common analysis with NMR and IR offers clear fingerprints for purity checks and batch validation.
Good labeling practices always help, especially for labs ordering specialty chemicals that carry liability concerns. Standard product expectations call for minimum purity above 98%. Moisture content often gets reported under 1%, with identification by both melting point range and spectral data. Labels need to show not just the batch and lot number, but clear manufacturer contact details to support traceability requirements. The Global Harmonized System (GHS) for chemical labeling flags the sodium salt as low-hazard, but documentation with safety data sheets is still required for import or university procurement.
I learned early in my career that shortcutting preparation steps often means trouble later, especially when building ester-sulfonate compounds. The typical route for this sodium salt starts with hydroxyethanesulfonic acid reacting with benzoyl chloride in the presence of a base, usually sodium hydroxide or similar. Controlling the pH and temperature through this step is key—if side reactions sneak in, purification gets much tougher down the line. Crystallization finishes up the isolation. Some factories add an extra recrystallization for higher purity demands, especially when the material heads for pharmaceutical testing.
Colleagues in custom synthesis talk about the importance of reliable reactivity. Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate stands out because the sulfonate group acts as an effective leaving group under mild conditions, opening the door to a wide range of substitution and coupling reactions. Benzoyl groups lend stability, but can also be swapped out for custom modifications at a later step. This flexibility lets chemists move beyond one-size-fits-all reagents. In practice, labs use the compound not just for direct sulfonation but also as an intermediate to build more complex sulfone derivatives or for the activation of alcohols in multi-step organic syntheses.
This chemical goes by more than one name, causing occasional headaches in lab logistics. Alongside the systematic name—Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate—sales reps call it Sodium Benzoyloxyethanesulfonate, or even SBHES in shorthand. It never hurts to double-check spectral data and CAS numbers before completing a purchase, avoiding substitutions or off-spec deliveries. Pharmacopeias and reagent catalogs stick close to IUPAC conventions, but I’ve seen academic papers lean on abbreviations that can add to the confusion.
Direct exposure risks don’t hit the levels seen with heavy-metal salts or strong acids, but basic PPE protocol applies for this sodium salt. Dust inhalation and hand-to-eye contact during weighing or transfer call for gloves and goggles. I’ve seen some older material safety data sheets that flag respiratory irritation risks, especially with large-scale handling, but routine lab practice keeps exposure in check. Emergency management focuses on spill containment and waste neutralization, both of which follow standard salt disposal methods. Adoption of ISO 9001 practices among specialty chemical manufacturers keeps batch traceability, purity checks, and recall readiness on track.
The main action happens in organic synthesis labs and specialty materials development. I watched this compound gain momentum among pharmaceutical companies tackling selective functionalization on aromatic compounds—where small performance gains turn into big advances. Water treatment companies and specialty polymer manufacturers sometimes pick up this sodium salt for its sulfonating action and ease of purification. Custom surfactant development and advanced coatings work also dip into the reagent, especially where precise property tuning is required. Academic research projects, especially those driving green chemistry or looking for clever synthetic shortcuts, draw on this sulfonate for both proof-of-concept and scale-up.
Scientists look for new pathways to make specialty chemicals safer, faster, or with less environmental impact. Small-scale pilot studies over the last decade have explored greener solvents for this synthesis and alternative benzoyl donors that could cut out hazardous intermediates. Bench-scale testing in custom synthesis projects often uncovers unexpected reaction selectivity or side-product challenges; more researchers study these quirks, feeding back knowledge into practical production. Patents pop up around process improvements, especially as the pharmaceutical and advanced materials fields stake out specialized derivatives. Some R&D labs even look into enzyme-catalyzed versions, seeking energy savings and fewer byproducts for mass production.
Like any chemical, risk follows exposure, and early-stage toxicology studies focused on both acute and chronic effects. Most findings point to low oral and dermal toxicity for the sodium salt itself. Some animal studies track typical sodium load responses, while mutagenicity screens for the aromatic benzoyl group stay negative under ordinary use conditions. Workplace exposure limits developed by regulatory agencies build on these data. Routine waste disposal practices—especially in pharmaceutical manufacturing—require neutralization and proper documentation, but the compound hasn’t drawn the red flags seen with organophosphate or halogenated intermediates. I make a habit of reviewing new data when it comes in, but so far, standard risk management practices work well for industrial and lab users.
Demand for more selective and less environmentally hazardous reagents keeps the spotlight on specialty sulfonates. Ongoing process innovation could support broader adoption, especially in sectors like precision medicine and advanced materials. I expect to see further automation and scale-up of green synthesis routes, as well as more affordable derivatives feeding new applications. End users continue to push for safer, more reliable intermediates in both research and commercial manufacturing. As the global market for high-purity synthetic precursors expands, Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate stands ready to meet new challenges, supporting industries as they adapt to ever stricter regulatory and product performance demands.
Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate isn’t some household staple, but it does pull its weight in certain industries. In the cosmetics and personal care aisle, this compound takes a role as a specialty ingredient in several formulations, especially for products that aim to cleanse or condition. Its chemical structure lets it function as a surfactant, helping to lift dirt and oils off skin or hair. You’ll find this approach in some shampoos, facial cleansers, and even a few specialized medical cleansers.
Outside the bathroom, this compound finds work as an intermediate in the making of other chemicals. Chemical manufacturers value the molecule’s reactive nature—there’s a spot where it can attach to other compounds, which makes it useful in the synthesis of more complex materials. Chemists appreciate how this contributes to the efficient production of dyes, pharmaceuticals, and sometimes cleaning agents.
Working in product safety, I’ve seen how detailed ingredient lists have become, and it’s clear folks want transparency. Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate raises a key question about safe exposure. While many surfactants do their job without causing harm, each new chemical needs a close look. Safety studies on this particular one remain limited compared to long-established surfactants like sodium lauryl sulfate. Animal toxicity data and reports of irritation haven’t caught up to those bigger names. Brands using it need to keep a sharp eye on patch testing and consumer feedback, especially with sensitive-skin products.
If a formula lists this compound, and someone’s prone to allergies or sensitivities, the lack of deep, long-term safety data can turn into a worry. Knowing how some ingredients can stay on the skin for hours, people want to trust that they won’t face rashes or long-term effects. Regulatory bodies like the European Chemicals Agency and the US Environmental Protection Agency expect thorough evidence of safety, especially for ingredients used across a wide age range.
Modern consumers recognize that what washes down the drain doesn’t just disappear. Surfactants have a history of lingering in water supplies if they don’t break down quickly. The persistence and breakdown rate of sodium 2-benzoyloxy-1-hydroxyethanesulfonate in the environment remain a patchy area of study, so using it at scale without more research could spell future headaches. Water quality monitoring teams worry about these newer chemicals slipping past standard filtration. Chemical persistence matters because it can affect aquatic life, even at low concentrations.
Another aspect: workers who handle concentrated forms of this compound in manufacturing deserve proper safety training and equipment. Accidental spills or dust can trigger respiratory or skin irritation, so protective measures aren’t just paperwork—they prevent real injuries. I’ve seen factory audits where lack of proper handling protocols led to increased absenteeism from skin irritation alone.
Manufacturers can commit to full safety testing and open reporting of any new findings. Third-party certifications—such as those from the Environmental Working Group or independent toxicologists—help set consumer minds at ease. Development of biodegradable alternatives and greener processes can lessen this compound’s potential impact on waterways. That might mean reformulating old favorites or investing in waste treatment upgrades.
For consumers, reading ingredient labels and reaching out to brands for information makes a difference. Pushing for transparency and evidence-based sourcing encourages companies to prioritize both safety and environmental responsibility. In my own work, I’ve seen that sustained consumer interest leads to real change in how ingredients are selected and monitored. As the science catches up, informed choices offer the best path forward.
Names in chemistry can feel like poetic riddles. Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate sounds complex, but breaking it down can reveal a lot. Sitting at its core, you find an ethanesulfonate backbone, which holds a hydroxy group at the first carbon and a benzoyloxy substituent at the second carbon. The sodium pops in as a counterion, balancing the negative sulfonate charge. In real terms, this means the molecule brings together a water-loving sulfonate, a sticky benzoyl group, and a hydroxy unit, all in one tidy package.
The detailed chemical structure looks like this: the ethanesulfonate chain has a sulfonic acid group (-SO3-) at one end, with a sodium ion attached. The hydroxy group sits right next door on the first carbon, easy to spot. Holding onto the second carbon, you’ll find the benzoyloxy group—essentially a benzoic acid group merged via an oxygen bridge. Chemists often use condensed notations, so expect to see this written as: NaO3S-CH(OH)-CH2-OCOPh.
As someone who has spent years working with chemical reagents, I’ve seen the difference a subtle structural twist can make. The sulfonate tail ensures high solubility in water, making the molecule easy to handle in labs or industrial processes. Industries dealing with pharmaceuticals or specialty chemicals count on such solubility for both formulation and application steps.
The benzoyl ester piece does more than just look good on paper. It carries the aromatic ring's stability and can affect how the compound interacts with enzymes or other molecules. In synthesis, aromatic groups provide new handles for chemical reactions. It gives scientists creative options for targeting specific biological or industrial needs. The hydroxy group, sitting close by, can form hydrogen bonds or take part in further reactions. A molecule with this architecture blends flexibility, reactivity, and handling ease—qualities you rarely get in one shot.
Such molecules often find themselves tested as intermediates for preparing active pharmaceutical ingredients. Clean synthesis and minimal waste rank as top priorities for modern labs. Compounds with sulfonate and hydroxy groups can help, as they dissolve well and give straightforward separation options. Plus, the benzoyloxy group opens a door for selective deprotection or further transformation with standard lab chemistry.
There’s a catch, though. Sulfonate compounds can sometimes escape water treatment plants and reach the environment. Regulatory bodies like the United States EPA require manufacturers to disclose potential hazards and follow good manufacturing practices. Careful documentation, disposal planning, and continuous risk evaluation play huge roles. In my time handling specialty chemicals, transparent labeling and rigorous training made a difference. Without these, a simple mishap could turn a helpful compound into a real environmental headache.
Industry, academia, and regulatory groups work together more now than ever. Shared knowledge about the structure and reactivity of specialty chemicals like Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate keeps us all safer and more productive. Encouraging ongoing research, tighter collaboration, and better risk communication leads to smarter use of these clever molecules. Newer guidelines help chemists navigate the gap between discovery and practical use, making science work for everyone involved.
Safety data, clear instructions, and swift feedback loops are tools any lab or plant should adopt if they hope for progress. Open discussion about potential hazards, plus a commitment to greener chemistry, keeps the focus sharp both in and out of the laboratory. Every part of a molecule’s structure tells a story—tuning in can move science forward in truly responsible ways.
Safety never gets old when dealing with chemicals that might end up in food or consumer products. Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate sounds like something you’d only see in a research lab, but lines between industrial, cosmetic, and dietary uses get blurry sometimes. Doubt comes up whenever an unfamiliar compound enters the spotlight—especially if someone suggests it could have a spot in things people eat, drink, or put on their skin.
Scientists judge safety by looking at a bunch of things: animal studies, human data, toxicology reports, and even long-term stories from countries where a chemical has floated around for years. The FDA, EFSA, and International Agency for Research on Cancer set rules and review data each time a company wants to introduce something new. Companies then hand over piles of documents showing how their product behaves in a petri dish, in an animal, and if available, in humans. Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate, though, doesn’t draw up results in the same way as older, better-known compounds.
Looking through published safety studies, nobody bumps into broad clinical trials or years-long population reviews of Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate. If a compound stays mostly in the industrial or research setting, large-scale safety data doesn’t usually appear. Whenever an ingredient has only petri-dish or animal-lab data, people ought to tread carefully. Animal testing helps draw a rough map but rarely fills in all the details for humans. Some chemicals that look harmless in mice or rats turn unexpectedly harmful in people.
“If it’s not approved by the big health agencies, and if decades of safe use by people aren’t on the books, I want to see serious proof before anyone adds it to human food or personal care products.” My background studying chemical risk gets me asking for hard numbers and “after-the-fact” real-world usage data. I wouldn’t advise a friend or family member to try something unless clear, high-quality research stands behind it.
Clear information remains tough to find for this compound. Without that, companies and regulators can’t spot risks before they hit the real world. Chemicals sometimes pass early toxicity screenings but then show hazards when millions encounter them over time. Strong regulation keeps risk lower, though history shows loopholes and oversight lapses invite mistakes.
Everyone has seen surprise recalls or lawsuits years after a product hit the market—think of issues with early plastics, talc powders, or certain artificial sweeteners. What keeps people safer isn’t just good science, but also real transparency, open risk communication, and a willingness to adjust. Agencies like the FDA demand detailed toxicology profiles and reserve approval for cases with clear benefit outweighing potential harm. Consumers deserve that diligence, especially when few real-world studies exist.
The people most at risk are those who might be exposed to new chemicals before independent science weighs in. A solution isn’t just more regulation, but also more open sharing of chemical safety testing by manufacturers, and more funding for independent research. Pushing for peer-reviewed studies, publication of negative results, and full disclosure of funding sources builds trust.
Looking out for long-term health takes relentless skepticism and a demand for evidence over marketing claims. Until robust data emerges for Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate, my experience pushes me to urge caution—not only for myself, but for the broader public, too.
Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate doesn’t pop up in everyday conversation. For those who handle chemicals, its name means a strict approach to storage. Improper handling leads to degraded quality or even safety hazards. My years working alongside research chemists taught me: a chemical’s temperament shows up in how it reacts to air, light, and temperature swings. Manufacturers and lab managers who don’t take the extra care risk damaged product and dangerous situations.
Moisture can turn a bag of chemical powder into an unpredictable mess. Most sulfonate salts, like Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate, draw in water from the air. Before you know it, clumps start forming, the material cakes up, and the purity drops. In some cases, unwanted side reactions might start up just from exposure. Handling this compound in a dry room or, better yet, under a dry inert gas like nitrogen, keeps problems out of your way.
Low temperatures go a long way. On a hot day, the wrong shelf could make chemical stability evaporate. Manufacturers usually recommend storing this material at room temperature, ideally below 25°C. In places with hot summers or poor ventilation, chemical cabinets with built-in climate control work like a safeguard. Refrigeration isn’t required, but sudden temperature changes can lead to condensation, so the room’s environment should stay consistent.
Some chemicals react to light even if the changes happen slowly. Storing Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate away from direct light helps cut the chances of photodegradation. Amber glass containers or opaque bins limit light exposure. In labs where fluorescent lighting runs all day, keeping containers shut does just as much good.
Mixing up containers often leads to disaster. Once, a coworker stored a sulfonate near bleach. Vapors mixed, and the chemical degraded faster. Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate doesn’t play well with strong oxidizers. Always use separate cabinets for incompatible chemicals. It’s a step that saves lives, not just lab budgets.
Labeling isn’t just a box to tick for lab inspections. It’s a habit that kept me out of trouble. Always write the product’s full name, concentration (if it’s in solution), and date received. Unexpected changes in color or texture mean it’s time for a closer look at the whole batch. SDS (Safety Data Sheets) give specific guidance on storage, such as ventilation or fire safety. Checking the data sheet before placing the compound in the stockroom lets you spot non-negotiable requirements like flame-proof cabinets for certain organics.
Accidents tend to spring from shortcuts. General storage advice for laboratory chemicals—cool, dry, well-ventilated areas and airtight containers—applies to Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate. These steps keep contamination risks down and ensure materials perform as needed in reactions or product formulations.
The people working with chemicals drive home the most important lesson: never treat storage instructions as optional. Automation can help here. Inventory systems notify staff when temperature drifts out of range or humidity creeps up. Regular training makes sure new team members respect storage standards. This approach, matched with clear labeling and accessible safety data, lets science move forward safely and efficiently.
Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate carries a name that sounds daunting to most, but its identity comes through in both the lab and on paper. Usually obtained as a crystalline powder, it appears white, clean, and straightforward, much like other members in the sulfonate family. If you’ve ever handled it, you remember its fineness and how it disperses in water, giving no sign of grittiness or insoluble bits.
Chemists appreciate substances that mix well with water. This sodium salt dissolves rapidly, which allows for fast and controllable reactions in solution. Since it features a sulfonate group, water-based systems welcome it—think detergency, personal care formulas, or pharmaceuticals that must avoid lingering clumps or undissolved components. It often comes with a melting point exceeding 100°C, so it handles shipping and storage without packing itself into lumps during summer heatwaves.
By comparison to less stable compounds, its aroma and volatility rarely stand out. You won't catch a whiff of anything strong or unpleasant in a typical lab, making it safe for daily work without causing headaches or safety flags. The powder sticks around the lab environment well enough, but dust control remains important. Over time, moisture from humid air can affect flow, creating a cake, so dry, air-tight jars make practical sense for anyone who stores it.
A prominent factor is its dual role: The benzoyloxy group can take part in gentle ester-like reactions, while the hydroxy and sulfonate groups increase its reactivity in water-driven systems. Its sodium ion offers high solubility—a trait that directly relates to how it functions industrially. Once in solution, this compound won’t fight pH swings aggressively; it generally sits comfortably on the neutral-to-slightly-basic side.
The molecule resists oxidation under normal handling. You can keep it on the shelf, mix it with commonplace laboratory reagents, and expect the white powder to keep its original qualities for months. If someone did heat it strongly or add aggressive acids, decomposition could start, breaking away the benzoyl or hydroxy portions and liberating fumes. Most commercial uses steer away from extremes, focusing instead on its reliable dissolution.
Many see value in compounds that combine stability with a knack for blending into water-heavy formulas. Although not famous among household cleaning names, its backbone shows up in specialized processes: some pharmaceutical intermediates, some niche surfactants. Its lack of color and taste, paired with stability, lets formulators focus on the active ingredient without worrying about a cascade of unpredictable breakdowns.
Environmental impact also matters. Sodium-based compounds like this typically wash out clean, posing less risk to aquatic life compared to certain older, oil-heavy chemicals. While no one should dump its solutions directly down the drain, its breakdown products rarely stick around in the ecosystem the way heavy metals or chlorinated aromatics do.
Relying on imported fine chemicals leaves buyers exposed to shifts in sourcing and quality. Labs using this compound should always request certificates of analysis to check for trace contaminants, which can affect sensitive research. Consistent raw material sourcing stays key for pharmaceuticals in particular, where even small changes in purity can trouble an otherwise steady process.
One practical improvement: pushing for greener synthesis, cutting out unnecessary solvents, and recycling process water. Clean, responsible production routes already exist and can scale up to more industries if the effort holds strong. Buyers and producers alike have a role in keeping their eyes fixed on both safety and sustainable chemistry.
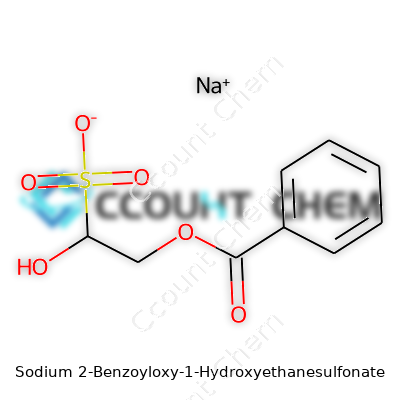
| Names | |
| Preferred IUPAC name | Sodium 2-benzoyloxy-1-hydroxyethane-1-sulfonate |
| Other names |
Sodium 2-(Benzoyloxy)-1-hydroxyethanesulfonate Sodium 2-benzoyloxyethane-1-sulfonate 2-(Benzoyloxy)ethane-1-sulfonic acid sodium salt Benzoic acid 2-sulfoxyethyl sodium salt BESNA |
| Pronunciation | /ˈsəʊdiəm tuː bɛnˈzɔɪ.lɒk.si waɪ hɑɪˈdrɒk.si ˈeθ.eɪnˌsʌl.feɪt/ |
| Identifiers | |
| CAS Number | 82945-43-5 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Sodium 2-Benzoyloxy-1-Hydroxyethanesulfonate**: ``` C1=CC=C(C=C1)C(=O)OCCS(=O)(=O)[O-].[Na+] ``` |
| Beilstein Reference | 1431586 |
| ChEBI | CHEBI:91242 |
| ChEMBL | CHEMBL2103838 |
| ChemSpider | 185624 |
| DrugBank | DB09293 |
| ECHA InfoCard | 100.195.216 |
| Gmelin Reference | 88454 |
| KEGG | C14327 |
| MeSH | D013480 |
| PubChem CID | 15585296 |
| RTECS number | WO2080000 |
| UNII | 38W8441OLM |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C9H9NaO5S |
| Molar mass | 288.27 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.49 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.2 |
| Acidity (pKa) | -2.0 |
| Basicity (pKb) | 1.63 |
| Magnetic susceptibility (χ) | −38.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.570 |
| Dipole moment | 2.98 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 386.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | B05CX04 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Lethal dose or concentration | LD50 Oral Rat 1310 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): >2000 mg/kg |
| NIOSH | NA239 |
| PEL (Permissible) | PEL not established |
| REL (Recommended) | 20~25°C |
| Related compounds | |
| Related compounds |
Benzoyl peroxide Sodium benzoate Ethanol Benzyl alcohol |