The roots of sulfonated aromatic compounds stretch back to the early part of the 20th century, a period marked by relentless odor of chemicals and the hiss of Bunsen burners in both academic and industrial settings. Sodium 2-Amino-4-chloro-5-methylbenzenesulfonate traces its ancestry to Germany’s dye factories, forged through painstaking trial and error that slowly revealed the relationships between structure and function in aromatic rings. Its core, a benzene ring with a sulfonate, methyl, chloro, and amino group, echoes the experimental boldness that shaped the modern chemical sector. I remember thumbing through battered catalogs from the 1970s, seeing sulfonates listed with cryptic abbreviations, most going unnoticed except by dye chemists or those working with specialty intermediates. Its development mirrored a period when efficiency and new functionalities meant better colors, water solubility for dyes, and the launchpad for pharmaceutical structures. Demand for well-characterized, high-purity aromatic sulfonates surged as analytical techniques improved, letting researchers tune properties for brand new uses.
Sodium 2-Amino-4-chloro-5-methylbenzenesulfonate stands as a fine, off-white to yellowish powder, drawing little notice by appearance. What sets it apart lies in the substitution pattern: an amino group at position 2, chloro at 4, methyl at 5, and a sulfonic acid at 1, neutralized by sodium. This specific arrangement bestows water solubility and a unique reactivity profile prized among both dye manufacturers and researchers hunting for building blocks with particular electron density features. Anyone who’s ever weighed out sulfonates for a batch run can attest to its easy handling, with a consistency and pourability that fits right in among solid organics. Typical packaging in airtight, moisture-resistant drums reflects the sector’s hard-won knowledge that moisture makes even the best batch clump or degrade.
This compound brings an unmistakable sharpness to the lab bench, crumbling under gentle pressure and dissolving with speed in water. With a melting point well above 200°C (decomposition), low volatility and a solid density, storage doesn’t generally prompt worry for accidental loss. It rarely emits strong odors. The electron-withdrawing sulfonate and chloro, balanced by the donating methyl and amino, impart a reactivity profile that allows selective coupling and diazotization. Anyone who has tried to reduce or oxidize similar benzenesulfonate salts knows they resist harsh treatment, requiring precise conditions when transformation is desired. Known for its photostability and resistance to light or air oxidation in storage, it remains a stable staple on laboratory shelves.
Good suppliers won’t skimp on documentation. A typical technical sheet points out a minimum purity level, usually in the 98% region, alongside information about related substances, moisture, and sodium ion content. Labeling cues chemists to avoid confusion with lookalike sulfonated anilines, listing the full IUPAC name plus well-known synonyms. Some manufacturers include spectral data, such as IR and NMR references, to help with batch-to-batch quality checks. Safety data lingers in the mind: labeling warns about dust generation and provides basic PPE advice, and the packaging material selection tries to balance chemical resistance and practicality.
The synthesis of Sodium 2-Amino-4-chloro-5-methylbenzenesulfonate offers a real lesson in methodical process design. Typical approaches start from methylchloroaniline, a molecule familiar to anyone who has worked a bench in an aromatic amine lab. Sulfonation usually happens first, often with fuming sulfuric acid or oleum, the reaction kept cold to avoid over-sulfonation or unwanted rearrangement. Neutralization follows, creating the sodium salt. The workup requires careful pH adjustment, sometimes under nitrogen to curb oxidation, before filtration collects the target. Recrystallization, if the process scale allows, bumps up purity for applications needing confidence in every lot. Cuts to water and salt consumption matter more than they once did, and increasing filtration speed reduces downtime for plant operators.
I’ve watched chemists try every trick in the book to coax new reactivity from this molecule. The amino group serves as a key handle: diazotization, followed by coupling reactions, unlocks azo dye synthesis with a spectrum of hues. The chloride at the 4-position resists displacement, but with the right catalyst or nucleophile, aromatic substitutions sometimes succeed. Sulfonate’s presence stabilizes the entire ring and blocks many positions from substitution, so chemists must plan carefully before embarking on modifications. N-alkylation and N-acylation work well under standard protocols, expanding utility for pharmaceuticals or specialty pigments. From time to time, researchers explore oxidation or reduction pathways, though practical applications have found limited commercial traction.
Reading through chemical supply catalogs, I’ve come across a bewildering array of product names: 2-Amino-4-chloro-5-methylbenzenesulfonic acid sodium salt, or its more compressed forms like "Sodium 2-amino-4-chloro-5-toluene sulfonate". European texts prefer names reflecting the aniline origin, while others list it under trade designations tied to dyes. Chemical Abstracts assigns it a unique registry number, streamlining regulatory communication. Old-timers still reference it by plant code, a practice frowned upon today for international shipments. Those working with regulatory filings must master the subtle differences among synonyms to avoid costly delays in customs or compliance checks.
Lab veterans recognize the need for clear guidance on proper handling. Dust generated by powders like this does pose risks if inhaled, even though the acute toxicity isn't especially high. Gloves, eye protection, and, for larger weigh-ups, dust masks avoid irritation. Spills clean up with a quick sweep or vacuum, and the waste rarely ends up classified as especially hazardous. That said, scale-up to tonnage brings with it the need for better air filtration, good ventilation, and regular safety audits. Process automation has done much to lower staff exposures. Local fire codes tend to treat such sulfonates as low risk, but careful auditors never ignore the sodium component or trace off-gassing during inadvertent heating.
Few materials stay stuck in just one niche. This compound found its first real calling in the dyes and pigments sector, where it serves as a backbone for colorants with excellent water solubility and lightfastness. Textile plants appreciated how it blended into dye baths without fuss, giving repeatable shade development. In more recent decades, the molecule’s well-understood reactivity pattern fostered interest in drug and agrochemical research. Many a PhD thesis has probed its potential as a building block for bioactive molecules, given the potential for further functionalization at the aromatic core. Specialty developers use it as a reference point in structure-activity studies, especially when exploring how neat substitution patterns change outcomes in both chemical and biological systems.
Across decades, sodium 2-amino-4-chloro-5-methylbenzenesulfonate gets ongoing attention in both academic and industrial circles. Teams look to tweak its substitution to push for brighter colors or more useful pharmaceutical intermediates. Enzyme-catalyzed transformations lately have gotten a hard look, with the aim of opening the benzenesulfonate realm to greener and more selective routes, cutting down on waste and energy use. Green chemistry goals continue to push for water-based processes and recycling of byproducts. In my own work, running side-by-side syntheses with classic acid catalysis and next-gen biocatalysis, the differences in yield and waste streams make a persuasive case for continued investment in R&D. Analytical chemists benefit too, since thorough characterization at each stage lets the industry maintain tight controls on impurity profiles, greatly increasing confidence among downstream users.
Data from animal and in vitro studies paint a picture of moderate toxicity, similar to other sulfonated anilines. It hardly ranks as a hazard in the crowded world of industrial chemicals but won’t pass muster in pharmaceuticals or food unless impurities get driven down to very low levels. Chronic exposure studies stretch back several decades, mostly reassuring, though regulatory agencies keep a watchful eye on potential links to delayed health effects. Any worker in the chemical industry appreciates the shift toward better monitoring and personal protection, with regulators demanding increasingly sensitive test methods. Environmental studies find the salt only modestly bioaccumulative, with water solubility assisting in processing or cleanup during waste treatment. Still, the aromatic core and amino group keep some regulatory concerns simmering in Europe and the US — careful documentation and transparent reporting have helped maintain its legal status in major markets.
Demand for specialty sulfonates remains strong as the search for innovative dyes, polymers, and advanced materials continues. Programmable chemistry and reactor automation may provide new pathways for this venerable intermediate to reach tomorrow’s medicines, sensors, and coatings. Growing regulatory scrutiny creates pressure for ever-cleaner processing, pushing producers to hunt for new purification steps and ways to reclaim byproducts not just for environmental reasons but cost savings, too. Advances in computational chemistry enable researchers to predict electronic and reactivity changes from subtle substitutions, charting more precise modifications and fewer failed attempts in the lab. The interplay of stricter environmental rules, rapid analytical advances, and creative new industrial uses promises to keep sodium 2-amino-4-chloro-5-methylbenzenesulfonate in the conversation for years to come. As supply chains get leaner and the appetite for tailored property sets grows, well-characterized intermediates with dependable quality take on even greater importance. From my own experience troubleshooting downstream failures, it’s never just about a single molecule but the whole raft of choices made along the route from raw material to finished product.
Sodium 2-Amino-4-Chloro-5-Methylbenzenesulfonate doesn’t sound like something you'd find in a kitchen cupboard, but its role matters in more ways than most realize. Its foundation lies in the world of dyes—serving as a key intermediate in the creation of vibrant colorants. For decades, industries have relied on this chemical to make reactive dyes, especially those that color cotton and cellulose fibers. That’s not just about blue jeans or summer T-shirts; it runs deeper into material science and textile sustainability.
Imagine a world where clothes keep fading, hospital uniforms don’t retain their distinct tones after a few washes, or the yellow curb lines vanish under sunlight. The chemistry that keeps color locked into fabric doesn’t happen by magic. Chemicals like Sodium 2-Amino-4-Chloro-5-Methylbenzenesulfonate serve as critical pieces in dye manufacturing. Its special structure brings three abilities—helping the dye anchor to fibers, letting color makers change dye shade precisely, and enabling large dye factories to scale production with confidence.
I’ve seen firsthand how production decisions hinge on quality and reliability. In textile mills, quality control managers look for dyes that hold up after repeated washing and sun exposure. If the dye intermediate misses the mark in purity or consistency, the whole batch risks ending up in landfills. The demand for strong, colorfast dye means chemists value intermediates like this one in their recipes.
Most of the global textile trade links back to Asia—India and China in particular—which together produce billions of meters of dyed fabric every year. They count on intermediates just like Sodium 2-Amino-4-Chloro-5-Methylbenzenesulfonate for the vivid reds and deep blues consumers expect. The knock-on effect trickles from textile workers through retail shelves, shaping trends and economics both.
While the dye world claims most of the demand, this compound can act as a base for synthesizing special pigments and markers, including reagents in some laboratory tests. A few pharmaceutical research groups have looked at sulfonated aromatic amines like this as potential leads during drug discovery, although no major drugs currently come from it. The main story stays rooted in keeping colors sharp and reliable for daily products.
A persistent snag emerges—how to use industrial chemicals like this one without hurting waterways or communities. Factories in countries with lax pollution controls have released sulfonated dye intermediates into rivers, turning them toxic and unsafe. The World Bank and several NGO reports document cases of dye pollution. The answer lies in building better treatment facilities and holding suppliers to safe waste disposal standards. Fashion brands can play a part by demanding transparent supply lines and backing partners who use modern, closed-loop manufacturing.
A responsible approach means investing in cleaner reactions and biodegradable dyes, an area chemists are exploring. Leaner processes often cut down on dangerous waste and energy use. As with most chemicals, transparency, strong science, and regular inspections offer the best shot at making sure necessary compounds like Sodium 2-Amino-4-Chloro-5-Methylbenzenesulfonate keep adding value without long-term scars on people and the planet.
Safety doesn’t live in a set of rules on a wall. It shows up in your daily habits—how you lift a heavy box, how you label a bottle, how you react when you catch a strange smell in the storeroom. At home and in workplaces, misunderstandings about product safety lead to accidents that hurt people and property. The stories we hear of chemical burns, fires, or poisoned pets all start with one step missed or a safety manual ignored.
Labels aren’t just legal paperwork; they help you work smarter. If a cleaner claims to zap every germ, glance closer. That fine print maps out risk. Look for hazard symbols. Know the difference between “corrosive” and “flammable.” Manufacturers include these details because past mistakes wrote the rulebook. Even a familiar product sometimes changes. Formulas change, packaging shifts—read each label, even if you think you know it already.
Gloves and goggles feel awkward, I get it. Sweating underneath a plastic apron never tops my to-do list. Still, burns on your skin or lungs aching after careless exposure land you in a far worse spot. In labs, shops, garages—protective gear guards against permanent scars and hospital visits. Real-world data backs this up: The Bureau of Labor Statistics found that injuries often trace back to skipped gear in routine tasks. There’s no badge of honor in toughing it out. Gear buys you another day to come home safe.
Tucking away a jug on a wobbly shelf or leaving something out "just for a minute" invites trouble. Products that react to heat, sunlight, or simple air contact can spoil or ignite. Safe storage means cool, dry, and always out of reach for children and pets. If a bottle leaks, store it upright and replace the cap securely. Store acids away from bases. Never stack flammable goods near stoves or heaters. In many cases, insurance claims and fire department records show the same pattern: poor storage turns minor spills into headlines.
Spills catch almost everyone off guard. I’ve seen coffee shop back rooms, school labs, even garages become accident scenes. Rushing to mop up a spill with paper towels spreads toxic residue, risking skin and lungs. Keep a spill kit wherever you store dangerous products. This should have absorbent material, gloves, and disposal bags. Train everyone who shares that space. Quick action only works if you recognize the risk and reach for the right gear without hunting for instructions.
Throwing leftover chemicals in the trash, sinking them down the drain—these shortcuts poison water and soil. Municipal waste services often provide disposal guidelines or dedicated collection days. Follow them, and ask questions if the directions confuse you. By disposing responsibly, you protect everyone downstream, both literally and legally. Fines stack up fast for illegal dumping, and cleanup costs can close businesses or result in criminal charges.
Each safety step starts with a personal decision. Companies create handbooks and procedures after learning expensive lessons. As a community member, worker, or homeowner, you become an expert over time—by observing, asking, and sharing what you learn. Take pride in getting it right; encourage others to do the same. Safe handling and storage never come down to luck. They happen when people make practical choices backed by facts and real experience.
Sodium 2-amino-4-chloro-5-methylbenzenesulfonate doesn’t roll off the tongue, but it shows up in chemical labs and some dye businesses more than most realize. The compound comes with a sulfonate group paired with an aromatic ring—a structure that says a lot about how it interacts with water and different solvents.
Sulfonate salts usually mix well with water, thanks to their ionic bonds. In real lab work, sodium 2-amino-4-chloro-5-methylbenzenesulfonate behaves just as expected from this family. After shaking a sample in distilled water at room temperature, tiny crystals vanish quickly, forming a clear solution. Published references back this up—estimates put its solubility above 100 g/L. That strong compatibility with water helps chemists make reactive solutions fast, reducing waiting times. For anyone making dyes or running syntheses, this level of solubility means less effort dissolving powders and a lower chance of precipitation ruining a batch.
Move this compound to a non-polar solvent—like toluene or hexane—and the story flips. You can shake, stir, or heat, but that white powder sits stubborn at the bottom. Over the years, I’ve found this pretty standard with sodium sulfonates. Ethanol and methanol sometimes show a little improvement, especially if water’s around, but the jump in solubility never matters much for practical work. DMSO and DMF, the heavy-duty polar solvents, can do the job if water absolutely won’t do. Still, unless a synthesis or separation process demands those solvents, most lab chemists stick with water.
In the world of dye chemistry, pharmaceuticals, and even wastewater research, the way a compound dissolves can make or break a process. Poor solubility forces buyers to order special grades or pre-dissolved forms—raising costs and lowering flexibility. When a sodium sulfonate dissolves as easily as table salt, workflows stay smooth. We don’t waste time fiddling with temperature or pH. That keeps safety up, since workers handle fewer chemicals and face less dust or accidents.
Some industries want less water in their products. If a company is blending organic solutions—paints, coatings, or even certain polymers—the lack of solubility in oil-like solvents creates limits. Surfactants and co-solvents often get added to force materials like sodium 2-amino-4-chloro-5-methylbenzenesulfonate into oily solutions, but finding the right blend takes trial and error. During my years supporting scale-up operations, we often ran pilot batches with various co-solvents, sometimes switching to a compatible salt form or tweaking the formulation. Keeping open communication flows between bench chemists and production techs helps spot sticky places before wasting large amounts of material. Bench testing on a small scale saves frustration down the line.
Clear data on solubility doesn’t just make work easier. It helps with storage—knowing a substance won’t “cake” or separate unintentionally. It also supports environmental safety. Highly water-soluble salts wash away quickly, so plants and facilities need proper filters and neutralization tanks to keep run-off under control. Regulations now expect discharge water to be well below certain thresholds. Testing and tracking these levels keeps companies out of legal trouble and helps protect community water sources.
Purity isn’t just a fancy lab term. Walk onto a factory floor or into any R&D lab, and you’ll see why purity matters every day. If someone asks for a chemical that’s “99.9% pure,” they aren’t just chasing numbers—they’re protecting processes, people, and products. Let’s get one thing straight: a small change in purity can mean the difference between success and a pile of unusable waste. Years ago, I watched a team scramble to trace an impurity that ruined an entire pilot batch. It set the project back months, cost thousands, and taught everyone involved how important clear information on chemical quality really is.
You’ll hear about grades like “technical,” “laboratory,” “pharmaceutical,” and “food.” Each one targets a different group—painters, professors, pharmacists, or producers. A paint shop ordering technical grade titanium dioxide won’t blink at trace metals most labs would call unacceptable. Meanwhile, a pharmaceutical company can’t budge on even slight impurities. Their decisions affect health and safety, and the regulations leave no room for shortcuts. People might think the difference lies only in price, but the paperwork, traceability, and batch control all get stricter as you climb the grade ladder. Trace contaminants that would barely make a difference in dirt removal or concrete setting can spoil results in a blood test or medicine batch.
Some suppliers toss out impressive numbers but skip the details. If the only thing listed is “>99%,” that omits a lot. What are the remaining unknowns? Is it sodium in the mix? Heavy metals? Solvents? One chemist told me about a time a “high-purity” order produced bright pink crystals instead of the desired white—residual iron wasn’t in the paperwork. Manufacturers and buyers deserve the whole story. The best suppliers break it down by specific contaminants and batch test results, not just global numbers.
It’s tough to separate solid vendors from sales pitches. Look for those who show real batch data, engage directly with questions, and can point to recognized standards like USP, ACS, or ISO for their grades. Organizations with a record of quality complaints or spotty documentation put both business and safety at risk. I always recommend getting third-party certification whenever possible, especially for any use connected to health, food, or environmental impact.
People sometimes gamble on off-grade or cheaper alternatives to shave costs. That kind of risk echoes through every stage of scaling up, from failed pilot runs to expensive recalls. Decision-makers should measure not just price but also reliability, available documentation, and the likelihood that a supply meets the bar every time. Setting clear specifications up front and insisting on certificates of analysis cuts down on costly surprises. I’ve learned that clear communication between buyers and suppliers solves confusion and uncovers hidden risks long before they reach the plant or lab.
In the end, chemical purity and proper grading impact more than just science—they shape outcomes across entire industries, and everyone from process engineers to end consumers feels the difference. Demand the data, expect the proof, and never settle for less than the full story.
A chemical like Sodium 2-Amino-4-Chloro-5-Methylbenzenesulfonate doesn’t travel well by accident. Plenty of labs and factories rely on it, especially in dyes or pharmaceutical work, so that means it travels by road, air, or ship. Like anyone who’s handled dry powders or specialty chemicals, you start to respect just how much fuss a little moisture, heat, or jarred packaging can cause.
Bulk drum or small bottle, you want rigid, airtight containers that won’t split. Polyethylene or high-density polypropylene can resist leaks and don’t react with the chemical. Cartons or double-layer fiber drums give a backup. I’ve seen corners cut in packaging to save a few dollars, and it always ends in lost product and risk. Don’t bet on single-layer bags—they tear open, powder escapes, and cross-contamination becomes a headache.
Humidity can ruin a chemical’s usefulness fast. After years working in shipping, a single overlooked pallet in a damp warehouse showed me how small lapses snowball into wasted inventory. Desiccant packs inside each container make a difference, and a liner keeps out humidity if the warehouse leaks. It’s common sense to seal every cap tightly, but adding tamper-evident shrink bands or seals—something a supervisor can spot at a glance—brings peace of mind.
Every person who handles a drum or jar must know what’s inside and how to stay safe around it. Big, bold labels in the local language list the proper chemical name, hazard symbols, and emergency treatment advice. More than once, poorly labeled cargo gets shoved in the wrong zone of a shipping depot, igniting confusion and possibly hazard. Proper labeling means less hesitation if a spill happens—someone grabs the right PPE fast.
Shipping regulations protect people and keep surprises to a minimum. The UN recommends specific packaging codes, and the Department of Transportation sets extra layers of rules for hazardous chemical transport. Fines for skipping rules can close a business; way more serious, somebody gets hurt or sick if shipments aren’t packed properly. I always pushed for up-to-date Material Safety Data Sheets and real training for loaders, because I’ve seen shortcuts end with a trip to the ER.
Stacking heavy packages wrong shifts loads and snaps container walls. I’ve relied on pallets locked with strong stretch film, never letting them pile too high. For shipping by air, chemical-resistant outer cases help in turbulence. On the road, sealed drums ride best lashed down and upright, so no tumbling or loose powder escapes if there’s a pothole or sharp turn.
From my own experience, shipments work best when all the links—warehouse, driver, shipper, receiver—know exactly how to check for leaks, document every step, and keep eyes open for mistakes. Routine checks for expiry dates, container condition, and inventory logs add accountability. Training everyone who handles the chemical—no matter their job—to act fast in an emergency, gives real results.
A good shipment is invisible: no spills, no confusion, no drama. Careful packaging and clear rules let Sodium 2-Amino-4-Chloro-5-Methylbenzenesulfonate arrive in top shape, so nobody down the line has to play catch-up or worry about safety.
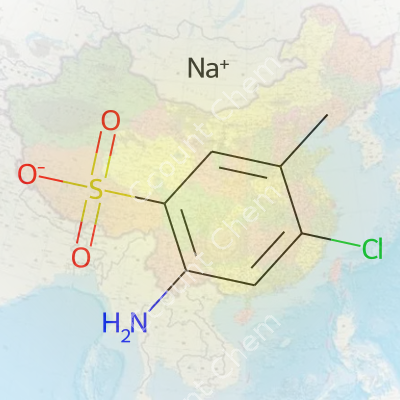
| Names | |
| Preferred IUPAC name | Sodium 2-amino-4-chloro-5-methylbenzenesulfonate |
| Other names |
Sodium 4-chloro-2-amino-5-methylbenzenesulfonate Benzenesulfonic acid, 4-chloro-2-amino-5-methyl-, sodium salt Sodium 2-amino-5-methyl-4-chlorobenzenesulfonate |
| Pronunciation | /ˈsəʊdiəm tuː əˈmiːnoʊ fɔːr ˈklɔːroʊ faɪv ˈmɛθɪlˌbɛnˈziːnsʌlˌfəneɪt/ |
| Identifiers | |
| CAS Number | 157245-05-1 |
| 3D model (JSmol) | `CC1=C(C=C(C=C1N)S(=O)(=O)[O-])Cl` |
| Beilstein Reference | 1369777 |
| ChEBI | CHEBI:87372 |
| ChEMBL | CHEMBL2103836 |
| ChemSpider | 23732810 |
| DrugBank | DB13826 |
| ECHA InfoCard | echa.europa.eu/substance-information/-/substanceinfo/100.029.085 |
| EC Number | 26283-44-5 |
| Gmelin Reference | 137528 |
| KEGG | C14345 |
| MeSH | D08.811.277.040.330.800.700 |
| PubChem CID | 157323 |
| RTECS number | DJ8925000 |
| UNII | E142R6MF8PR |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID4040966 |
| Properties | |
| Chemical formula | C7H7ClNNaO3S |
| Molar mass | 285.69 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.46 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa ≈ -2.8 |
| Basicity (pKb) | 6.06 |
| Magnetic susceptibility (χ) | -52.0e-6 cm^3/mol |
| Refractive index (nD) | 1.604 |
| Viscosity | 800 cP (20°C) |
| Dipole moment | 3.6 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 321.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -642.3 kJ/mol |
| Pharmacology | |
| ATC code | A16AX14 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261-P280-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 230°C |
| Lethal dose or concentration | LD50 oral rat > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | WF8575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium 2-Amino-4-Chloro-5-Methylbenzenesulfonate: Not established |
| REL (Recommended) | 13°C |
| Related compounds | |
| Related compounds |
Benzenesulfonic acid Sulfanilic acid 2-Amino-5-methylbenzenesulfonic acid 4-Chloro-5-methyl-2-aminobenzenesulfonic acid Tosyl chloride |