For decades, folks working in chemical industries have recognized the value of sulfonic acids and their salts. Sodium 1-hydroxyethanesulphonate may not enjoy the celebrity status of some other additives or building blocks, but its backstory stretches into the days when chemists started looking for alternatives to unpleasant, corrosive acids in cleaning and textile work. In my early days in the lab, safer, more stable acids made things simpler and less nerve-racking. The shift from raw ethanesulfonic acid preparations to sodium salts like sodium 1-hydroxyethanesulphonate cut down on dangerous spills and put a lid on uncontrolled reactions mid-process. The chemical’s debut as a viable option for complexometric reactions boosted its reputation fast, especially across Europe and Asia, where textile processing, dye preparation, and electroplating all demanded ever-better performance from basic chemicals.
Most people outside the chemical sector won’t recognize sodium 1-hydroxyethanesulphonate by its proper name, but chemistry teachers know its value in keeping reactions smooth and dependable. You might hear folks toss around synonyms like Isethionic acid sodium salt or sodium isethionate, though the substance goes by different trade names depending on where you are in the world. At its core, the value is pretty simple: it works as an efficient acid buffer, offers a mild surfactant action, and can help build chemical stability in pharmaceutical preps, personal care products, and food-grade formulations.
This compound grabs attention for being a white, odorless crystalline solid, soluble in water, and able to hold its own even under varied temperatures. I’ve had lab sessions where humidity spiked, but this salt didn’t turn to mush or cake up in the storage jar. Its melting point sits quite high, around 270°C without decomposition, which means it keeps its structure through a wide range of industrial heating and processing steps. With a molecular weight just over 126 grams/mole, its predictable solubility and non-hygroscopicity prevent processing hiccups, even at room temperature or below.
Precision stands front and center on product specifications for sodium 1-hydroxyethanesulphonate. Reputable suppliers document purity levels above 98%, with very tight limits on chloride, sulfate, iron, and heavy metals. Manufacturers selling to food and pharmaceutical suppliers must meet tough US and EU labeling requirements, including the chemical’s full name, grade, batch number, and explicit handling warnings right on the drum or bag. These transparency measures make it easy for everyone in the supply chain to know exactly what’s coming through the door, which cuts contamination risks. Allergen-free assertions and compliance with halal, kosher, or vegan standards also pop up more often as product documentation gets more rigorous over time.
Producers start by reacting sodium bisulfite with ethylene oxide under carefully controlled conditions, often relying on years of experience or rigorous automation to avoid runaway exothermic events. My old employer never cut corners during this stage, knowing a single slip could trigger plant-wide alarms. The steps involve neutralization, evaporation, and sometimes recrystallization to ensure high purity. By keeping impurities in check and adjusting pH throughout, the resulting salt meets stringent international regulatory standards, supporting its use in sensitive fields from food processing to pharmaceutical manufacturing.
Sodium 1-hydroxyethanesulphonate reacts smoothly with mineral acids, can serve as a ligand in complexometric titration, and sometimes finds application in transition metal chemistry. In personal experience, its reactivity opens up several doors for making gentle surfactants and acting as a precursor to novel ionic liquids. Researchers across Europe and Japan routinely experiment with chemical substitutions along its ethane backbone, tweaking it for better stability or improved bioavailability in new pharmaceutical applications.
Naming can get confusing. Textbooks and trade catalogs list it as sodium isethionate, 2-hydroxyethanesulfonic acid sodium salt, and a long string of other translations and abbreviations. Depending on the region, the labeling might favor Isethion, C2H5NaO4S, or simply “neutralized isethionic acid.” These synonyms create headaches for importers or new users, and mistakes here can mean purchasing the wrong compound altogether—something I’ve seen happen more than once in rushed procurement cycles. Strict documentation and cross-referencing remain critical.
In the industrial world, sodium 1-hydroxyethanesulphonate ended up in the “relatively safe” column compared to the more caustic acids it replaced. It carries a mild irritant warning and requires eye and skin protection, particularly with large-volume processing. Respirators remain standard in dusty environments. Proper storage protects the salt from moisture that can clump or degrade the product. Safety data sheets from North America and the EU stress strong ventilation in work areas and warn against mixing with strong oxidizing agents. Years in the industry make clear that written protocols aren’t enough—the right safety training, drills, and up-to-date personal protective equipment keep production lines in good standing.
The salt’s role stretches far: it stabilizes bath chemistry in electroplating shops, boosts mildness in shampoos and bath gels, and acts as a buffering agent in intravenous injectable drugs. In water treatment facilities, it finds use as a biodegradable sulfonate that breaks down without harming aquatic life—a trait becoming more important with tightening environmental rules. Its low toxicity profile makes it an option for sensitive pharmaceutical and cosmetic products, while in dye manufacturing it keeps the color chemistry robust and consistent. For smaller players like boutique cosmetic producers, sodium 1-hydroxyethanesulphonate comes in at a price point that doesn’t kill margins, and avoids heavy regulatory burdens linked with harsher alternatives.
Lately, R&D teams dive deep into modifying sodium 1-hydroxyethanesulphonate for bio-based surfactant applications, looking to replace petroleum-derived detergents. Pharmaceutical development keeps an eye on its use as a stabilizer in tricky injectable formulations. Academic chemists have driven fresh work on its capacity as a chelating agent, especially in green chemistry and catalysis. I’ve attended conferences where prototypes for isethionate-derived polymers made a splash, generating buzz about new bio-compatibility claims and improved breakdown in the environment.
Toxicologists and regulatory bodies highlight the low acute toxicity of sodium 1-hydroxyethanesulphonate. Chronic exposure studies on lab animals haven’t thrown up major red flags, and environmental fate tests show it degrades to safe byproducts in municipal wastewater systems. Animal testing shows few, if any, bioaccumulation concerns and it misses the lists of restricted substances published by major regulatory agencies. Still, some caution sticks around—overexposure or ingestion in large quantities can cause stomach upset or skin rashes, most frequently seen among workers handling bulk material without gloves. Responsible manufacturers continue to update guidance on handling and disposal as more data flows in.
The future for sodium 1-hydroxyethanesulphonate remains bright, shaped by the shift toward safer chemicals in consumer goods and ongoing calls for biodegradable, gentle alternatives. Demand follows the trend toward hypoallergenic personal care and growing pharmaceutical applications, as formulators see value in low-odor, non-reactive additives that don’t tip product stability out of balance. Innovations in “green” electroplating and water softening open new commercial doors each year. Small research groups keep exploring new uses, buoyed by regulatory support and market demand for alternatives to harsh chemicals. As an old hand in the chemical sector, I see more companies moving this way, anticipating customers who want gentle, earth-friendly solutions without giving up quality or performance.
Sodium 1-hydroxyethanesulphonate isn’t a name that shows up in everyday chatter, but it connects quietly to a lot of familiar processes. Most folks would never see it on a store label or at a supermarket, yet the chemical shapes products and processes used daily. People in manufacturing, medicine, and even agriculture cross paths with it, though most would never know.
Factories use sodium 1-hydroxyethanesulphonate mainly as an additive for making things run smoothly. For example, textile workers rely on it to keep dyes stable and to help colors stick to fabrics evenly. I’ve seen the trouble that pops up in a dyehouse when fabric colors turn out patchy or fade quickly. Keeping color fastness strong isn’t just about having a quality dye. You need things like this chemical to make sure the bath stays balanced, with fewer streaks and surprises.
This chemical also gets tapped as a component in cleaning products. Surfactant action keeps dirt on the run, and the sulphonate group shines here by breaking up greasy residues and rinsing them away without leaving scummy traces. Cleaning factories know how much headache builds up if residue clings to surfaces or equipment. Everyday cleaning products might include it for the same reason, working quietly between the lines of an ingredients list.
Hospitals and clinics need strong, stable solutions for disinfection and medical prep. Sodium 1-hydroxyethanesulphonate sometimes enters the picture here as a buffer, helping maintain the right pH in medical fluids. That pH balance plays a real-life role in wound care, making sure solutions used in rinsing or cleaning don’t irritate skin or interfere with healing. Years ago, I worked with nurses managing stubborn wounds, and every edge can make a difference when a patient’s recovery feels stuck. Stability in a wound wash solution can mean less guessing and more predictable care.
The pharmaceutical industry looks at this compound for its ability to keep drugs stable during manufacture and storage. Some medications, especially those in liquid form, benefit from a chemical that keeps the formula reliable over time. Patients who count on these drugs for daily needs rarely see the long string of names in an ingredient list, but skipping chemical stability risks spoiled medicine and lost trust.
Cleaning up water has never felt more urgent. Effluent from factories can threaten rivers, while municipal water supplies struggle with buildup from minerals and residues. Sodium 1-hydroxyethanesulphonate steps in as a chelating agent, helping lift out minerals like calcium and magnesium that clog pipes and foul water lines. Once, I saw what scale can do in a factory: machines shutting down, pipework sacrificed to stubborn crusts. A strong chelator means fewer repairs, cleaner lines, and water systems that last longer.
Every chemical brings benefits and questions. How much lands in rivers? Are workers protected in plants? Industry shifts toward greener solutions and stricter standards for additives. Manufacturers and researchers keep searching for ways to use sodium 1-hydroxyethanesulphonate responsibly, weighing what it gives against its impact at the other end. Better filtration, more transparent sourcing, and regular assessment matter for those committed to both smooth production and a healthier environment.
Sodium 1-hydroxyethanesulphonate, better known in some places as sodium isethionate, shows up a lot in labs and factories. Surfactant producers, researchers, and cleaners all work with it in some way. This compound draws attention because of its reactivity and the way it can irritate eyes, skin, and airways. It won’t rust steel panels or create fumes like stronger acids, but nobody leaves the bottle open on the bench. If you’ve ever splashed pure powdered sodium isethionate onto your arm, you know it tingles and burns after a few seconds, especially on broken skin. My own hands stung for hours because I brushed off residue without gloves one busy morning in a formulation lab.
The label makes it clear: goggles and gloves every time. You won’t find a chemist or factory operator taking shortcuts here. Even in a hurry, nitrile or neoprene gloves block the dust. Splash goggles stop granules from bouncing into your eyes, which hurts much more than it sounds. In cramped or drafty spaces, a face shield becomes important. Respiratory masks aren’t optional in places where powder floats around, especially if you work with it every day. Without proper protection, lungs might feel scratchy and sore, and coughing often follows. Long-term contact with powders like this can risk respiratory or skin allergies, so clean-up and PPE do more than just keep you comfortable in the moment.
Keeping sodium 1-hydroxyethanesulphonate dry helps prevent clumps or dangerous slips. Tightly sealed containers with good labeling cut out confusion and accidents. Humidity makes the powder sticky, which leads to spills, slippery benches, or awkward scooping situations. Any chemical that pulls in water from the air changes its properties over time, and this can surprise the next shift. I’ve seen someone open up a reused jar only to find it caked beyond saving, wasting a whole batch. That day the spill almost sent a colleague sliding across the lab floor.
Broom and dustpan work for dry spills, followed by a damp cloth—never blast it with compressed air or a dry brush. Heat or flames need to stay far away from clean-up zones. The powder isn’t flammable, but fine dust in the air presents an explosion hazard, same as flour mills or sugar factories. Used material or wash water needs collection in special bins, not down the regular drain. Proper waste disposal lines up with environmental regulations and respects the water table. As someone who used to manage chemical waste logs, I can tell you shortcuts here eventually find their way back to you—and the environment.
Good protection starts with people knowing how to work safely. Training helps everyone understand why PPE matters, how to handle the product, and what to do when things go wrong. Clear safety instructions, emergency showers, and well-marked exits build a sense of confidence. New team members pick up tricks from old hands—like never opening a new drum solo, and always checking for powder on your shoes. Experience teaches more than a label ever could. Ensuring safety every step of the way, from delivery to disposal, keeps everyone healthy and productive.
Sodium 1-hydroxyethanesulphonate goes by a less fancy name in some circles: sodium isethionate. To get right to it, the chemical formula is C2H5NaO4S. This formula packs a punch, combining carbon, hydrogen, sodium, oxygen, and sulfur in a structure that shows up in a surprising range of products we use every day. Breaking it down gives a sense of how much development and science go into creating and understanding even a single ingredient in many formulas.
Working in product development, I have seen how one ingredient can change the whole feel of a finished item. With sodium 1-hydroxyethanesulphonate, you see its real power when making things that touch the skin. It softens water, which helps make items like liquid soaps feel smoother without stripping away moisture. The drive for milder, eco-friendlier ingredients has brought this compound up the ladder. Having a good grasp of the chemical formula means chemists know exactly what they are adding and how it reacts—not just in a test tube but in a bottle on a store shelf.
Many people worry about what’s in the products they use on their bodies. Pulling up a label and running into “C2H5NaO4S” might sound intimidating, but that formula opens the door for proper research. Being able to point to peer-reviewed sources or trusted chemical databases reassures buyers. The European Chemicals Agency and U.S. Environmental Protection Agency both provide breakdowns on ingredients like sodium isethionate, helping people look past the jargon and see safety steps or regulations in place. Knowing the formula lets safety organizations trace potential interactions or risks, an important step in public health protection.
Over the last decade, shoppers have started looking for shorter, simpler ingredient lists. Sodium 1-hydroxyethanesulphonate fits into that trend since it can replace harsher additives and helps limit unnecessary chemicals. Ingredient transparency keeps moving forward, both from demand and regulatory pressure, putting the spotlight on reliable chemical identification. Hyphenated, easy-to-search formulas support this, breaking down barriers that used to sit between the consumer and their choices. More accurate labeling means fewer worries about hidden allergens or undisclosed manufacturing steps.
As research grows, using well-defined ingredients like sodium 1-hydroxyethanesulphonate keeps manufacturers honest about quality and consistency. Controlling input at the formula level improves batch-to-batch stability and lets companies troubleshoot any issue faster. During a stint in manufacturing, I learned firsthand that being able to trace every component back to its chemical signature beat any guesswork. This reliability means cost savings and fewer product recalls—something companies, consumers, and regulators can all support.
Chemists keep looking for ways to make essentials safer and more environmentally sound. Sodium isethionate offers a route away from more aggressive surfactants. Some brands have started promoting its use to show commitment to safety and sustainability. Continuous testing, open sharing of data, and investment in greener processes will keep this momentum going. Cleaner, clearer formulas create peace of mind and open the door for new advances, especially in personal care and cleaning products. The simple formula—C2H5NaO4S—reminds us that a handful of atoms, arranged just so, can shake up whole industries for the better.
I’ve spent some years in materials warehouses, walking rows packed with containers full of chemicals, watching how easily a simple oversight can go sideways. Sodium 1-hydroxyethanesulphonate, a mouthful on the shipping form, looks innocent enough at a glance. It helps a lot in textile, pharmaceutical, and cleaning product manufacturing. But it’s never a good idea to treat any industrial chemical like sugar or salt. Safety takes a bit of discipline and a touch of respect for what’s sitting on your shelves.
Moisture and chemicals don’t play nicely together. Water sneaks in and before you know it, you have clumping or, worse, unexpected reactions. I learned quick that you want Sodium 1-hydroxyethanesulphonate in a spot where the air stays dry. I’ve seen rusty shelving and sticky floors in forgotten corners; that’s trouble brewing. Stick to clean, sealed containers—polyethylene drums or triple-sealed bags do the trick. Once a bag is opened, if you leave it in a humid space or let the seal get sloppy, the contents start absorbing water from the air. That weakens product quality and can cause caking, making headaches for anyone measuring out precise doses.
Many chemicals don’t scream danger until they get too warm or catch a bit too much sunlight. I always tell new hires: shade is your friend. Direct sun on storage rooms or supply docks can push temps higher than you’d guess. If the room gets stuffy or hot, degradation speeds up. Product breaks down, performs poorly, or forms odd clumps. Think ground-level storage in a cool, shaded spot, never right by a window or on the top shelf nearest the roof.
I remember a time someone stored cleaning acids beside a bulk bag of Sodium 1-hydroxyethanesulphonate, thinking, “It’s just another chemical.” Mixing incompatible materials can cause runaway reactions, toxic fumes, or ruined goods. Never let acids or oxidizers get near it. Storage charts exist for a reason, keep them posted on the wall and check them. If your warehouse has a chemical-compatible storage area, make use of it and separate chemicals with solid barriers or clear labeling.
Messy shelves lead to mix-ups. Labels peel or fade in poor environments, and that’s a recipe for confusion. Every bag, drum, or bin should carry a clear label—chemical name, hazard warnings, date received. That way, anyone grabbing a scoop knows what they’re dealing with. Write the date you open any bulk container; it helps to track freshness and know what needs using first. No one wants to fumble with faded marker when seconds count.
I lock up the chemicals, even if it’s inconvenient. Never leave access open to people untrained in safe handling. Spills happen, and you want your spill kit nearby—absorbent material, gloves, and a sturdy waste container. Post emergency contact numbers near storage areas. If something goes wrong, no one is left searching in a panic.
I walk the rows myself, looking for leaks, tears, or signs of dampness. Any split bags or strange odors get flagged immediately. Training your crew to do the same cuts accidents and keeps the work environment safe. Keeping a close eye on storage conditions saves time, money, and stress down the road.
Sodium 1-hydroxyethanesulphonate, sometimes known in industry as sodium isethionate, pops up in a lot of formulations, especially among personal care products and cleaning agents. It draws attention not only for what it does on the shelf, but also for what it could do after the bottle is empty and tossed away. People are right to ask: Are we inviting risks with this ingredient?
Anyone who spends time reading ingredient labels probably has brushed up against sodium 1-hydroxyethanesulphonate in soap bars and shampoos. The substance acts as a surfactant - breaking up oils, helping things lather up. Research and regulatory reviews from agencies like the European Chemicals Agency suggest this substance has low toxicity for humans. It’s not considered a skin irritant at concentrations found in finished products, and long-term studies have not shown buildup in the body.
No chemical is risk-free, though. Eye contact, especially with a concentrated raw product, would cause stinging and discomfort. I’ve spilled soap base on myself during a homemade batch and can say this applies to many surfactant-type chemicals. This is a reminder to treat undiluted ingredients with respect, wear gloves, and avoid getting splashed in the eyes.
Allergies seem quite rare. Reports in the medical literature describing skin reactions trace most problems to fragrances or preservatives rather than sodium 1-hydroxyethanesulphonate itself.
Chemical runoff matters to fish, rivers, and soil health. Most people washing their hands or hair probably don’t realize those same suds rinse down the drain, finding their way to water treatment plants. Here’s where sodium 1-hydroxyethanesulphonate stands out: laboratory testing finds it breaks down easily in water. That means it doesn’t stick around the ecosystem for months piling up in aquatic life.
According to reviews from environmental scientists, concentrations in treated wastewater end up far below any level shown to cause trouble for fish or bugs living downstream. No evidence points to harmful bioaccumulation. For governments setting environmental guidelines, this earns the compound a tick in the “low concern” column. I look for these signs myself, especially after years of rinsing shampoo bottles into the sink and hoping ordinary routines don’t come back to haunt the environment.
The conversation about chemicals in household goods often lands in a space of trade-offs. Sodium 1-hydroxyethanesulphonate does its job without causing the kinds of health scares linked to some other surfactants. Still, every chemical comes with a footprint. Manufacturers testing alternatives should favor options with both low human toxicity and fast breakdown in the environment. There’s always room for reducing ingredients, dialing up transparency, and making sure new blends don’t drag hidden hazards into daily routines.
If you’re worried about product safety, the best habit is to stay informed, read up on reputable sources, and choose products with clear ingredient disclosures. Governments and companies need to keep up with new research, improve wastewater treatment, and reduce overcomplicated formulas where possible. Everyone stands to gain from products that clean up without leaving behind new problems.
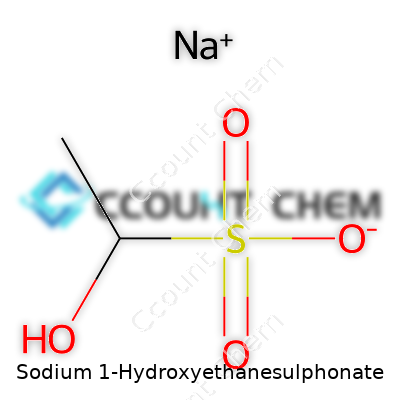
| Names | |
| Preferred IUPAC name | Sodium 1-hydroxyethane-1-sulfonate |
| Other names |
Sodium isethionate Isethionic acid sodium salt 2-Hydroxyethanesulfonic acid sodium salt Sodium 2-hydroxyethanesulfonate |
| Pronunciation | /ˈsəʊdiəm wʌn haɪˌdrɒksiˌɛθeɪnˈsʌlfəneɪt/ |
| Identifiers | |
| CAS Number | 1562-00-1 |
| 3D model (JSmol) | `C1=CS(=O)(=O)OCCO1.Na` |
| Beilstein Reference | 1438739 |
| ChEBI | CHEBI:135740 |
| ChEMBL | CHEMBL135354 |
| ChemSpider | 13847484 |
| DrugBank | DB14672 |
| ECHA InfoCard | 100.028.873 |
| EC Number | 240-012-7 |
| Gmelin Reference | 84958 |
| KEGG | C06861 |
| MeSH | D013480 |
| PubChem CID | 23665920 |
| RTECS number | WN5078000 |
| UNII | XR822Q1V4D |
| UN number | UN3265 |
| Properties | |
| Chemical formula | C2H5NaO4S |
| Molar mass | 126.13 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.48 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.53 |
| Basicity (pKb) | 10.08 |
| Magnetic susceptibility (χ) | -31.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4250 |
| Viscosity | Viscosity: 45.6 cP (25 °C) |
| Dipole moment | 5.7447 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 242.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -942.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1116 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V03AE03 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD₅₀ (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 5,900 mg/kg (oral, rat) |
| NIOSH | WN3850000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 35 mg/m³ |
| Related compounds | |
| Related compounds |
Ethanolamine Sodium ethanesulfonate 1-Hydroxyethane-1,1-disulfonic acid Methanesulfonic acid Sodium methanesulfonate |