Sodium 1,2-benzoxazol-3-ylmethanesulfonate didn’t come out of thin air. The path from raw chemical curiosity in early organic synthesis labs to commercially relevant compound reads like a story of how scientists work with what’s available until something useful drops out. Early research into benzoxazole derivatives in the mid-20th century pulled together lessons from heterocyclic chemistry and the growing understanding of sulfonate group functionality. Researchers tinkered, sometimes with little direct purpose at the outset, working through varied N- and S-alkylations, then discovering a set of unique physical characteristics and unexpected stability in water. Once chemists put together its sodium salt and surfactant-like properties, industry started to take a closer look.
It’s a white to off-white crystalline powder, fairly soluble in water, and often comes in high-purity forms for laboratory and specialty industrial use. API manufacturers and specialty chemical suppliers have included it among niche reagents. Companies usually source it for advanced intermediates and as a solubilizer or stabilizer in tricky synthesis projects, especially where water compatibility helps. The price point doesn’t put it out of reach for academic labs but pushes larger scale use closer to specialty manufacturing.
On the bench, sodium 1,2-benzoxazol-3-ylmethanesulfonate dissolves in water with little cloudiness, showing off its decent polarity. Melting points tend toward 240–250°C, with solid-state stability that resists regular temperature swings. The benzoxazole core brings aromaticity, but the sulfonate chain and the sodium counterion add hydrophilicity. You get a compound that doesn’t clump or cake up under standard storage, stays stable in neutral and mildly basic environments, and sees only minor decomposition under acidic or oxidizing conditions. Chemists often praise how cleanly it reacts compared to some other benzoxazole derivatives, showing predictability during downstream reactions.
Labels usually note purity above 98%, along with critical safety data like storage conditions—dry, room temperature, sealed away from acids and oxidizers. Sometimes, you’ll see extra batch data, synthesis route, even particle size or flow characteristics for those with automated handling needs. Some suppliers track trace metals and other potential contaminants. Regulatory codes follow global standards, using either the US or EU-specific chemical inventory listings to ensure buyers know the legal standing of the product. Lot numbers matter for tracking performance when used in regulated manufacturing or research projects.
Preparation typically follows a two-step process. Chemists first form 1,2-benzoxazol-3-ylmethanol from salicylaldehyde oxime through cyclization, then treat this intermediate with methanesulfonyl chloride under basic conditions. The resulting sulfonated derivative is neutralized with sodium hydroxide, purified by recrystallization from water or alcohol-water solvent mixtures. On the industrial side, carbon filtration can polish up the final product before drying. This route avoids harsh conditions, which preserves that sensitive benzoxazole ring. Each stage needs optimization, especially temperature control and pH monitoring, to avoid side reactions that could foul up downstream applications.
Research groups have run a variety of substitution and coupling reactions on this molecule. The sulfonate group opens doors for nucleophilic substitutions, while the oxazole ring allows for directed metallations and arylations. Cross-coupling reactions with palladium catalysts help scientists bolt on more complex aromatic groups. Sometimes, the sodium salt swaps out for potassium or lithium for tunable solubility or reactivity. Chemists may also reduce or oxidize the benzoxazole core, allowing the creation of derivative libraries for screening purposes. New work explores attaching the core structure to peptides or drugs for delivery or imaging applications.
In catalogs, it may appear under names such as sodium benzoxazolylmethanesulfonate, sodium 1,2-benzoxazole-3-methanesulfonate, or its CAS registry number. Some suppliers use in-house trade names or formulations, but these rarely stray far from the IUPAC standard for clarity in international trade and documentation. This avoids confusion when research gets published or products move between regulatory environments.
Handling stays straightforward if basic chemical safety protocols stick. It doesn’t give off vapors under normal conditions, and spills respond to standard water rinsing. SDS documents outline low acute toxicity but note irritation risk for skin or eyes, especially as a fine powder. Ventilation helps since even non-volatile organics can pose a nuisance dust risk. Industrial users install process controls to keep product away from strong acids or compatible organics—no sense risking unexpected runoff or exothermic surprises. Waste streams containing the compound go through neutralization and dilution before disposal in line with local regs. Training staff on direct handling and emergency response matters because experts can’t always predict an operator’s choices in a sticky situation.
You’ll find this compound in the toolkits of researchers chasing new heterocyclic drugs or agricultural chemicals, often acting as a water-soluble linker or reactive intermediate. In dye and pigment chemistry, some groups use it to build elaborate aromatic scaffolds or as a coupling agent. Water treatment and surfactant research also tap into its stability and charge properties, testing it as a possible anti-scaling additive. The direct consumer impact sits a step away, but without it, a bunch of our routine products and medicines would get more expensive or vanish altogether.
Teams in academic and industry settings keep probing new uses. Biochemists have started linking it to enzyme inhibitors or probes for fluorescence tagging in analytic chemistry. As combinatorial chemistry unpacks new drug leads, being able to graft the benzoxazole-sulfonate motif opens extra space in chemical libraries. Some companies now look at chiral variants to probe enzyme selectivity or try radiolabeling for imaging. Better synthesis routes, including flow and greener methods, make it attractive for those aiming at sustainability targets or worried about hazardous waste.
Toxicologists in pharma and environmental science labs haven’t flagged significant red alerts for this sodium salt. In rodents, oral and dermal exposure tends to pass with low absorption and minimal systemic impact, with the compound excreted rapidly in urine. Still, chronic and high-dose studies haven’t been as plentiful as with bulk chemicals, so caution wins out. Ecotoxicology shows even lower impact in aquatic life, likely due to rapid dilution and degradation. Most of the concern shifts to what happens if it gets altered or breaks down in mixed chemical waste, which remains an open topic for new study.
Biggest growth potential comes from its role in green chemistry and specialty drug development. Newer surfactants aim for strong water-compatibility with limited bioaccumulation, bringing sodium 1,2-benzoxazol-3-ylmethanesulfonate onto the shortlist for further testing. In drug discovery, attaching it to molecules for solubility tweaks or targeted delivery could offer new ways to address tricky diseases. As regulation tightens on legacy toxicants and persistent organics, any compound with a stable track record and evidence for benign breakdown products gains momentum. Where big data and AI meet chemistry, researchers model substitutions on the oxazole framework as tools for exploring bioactivity, working out in silico what takes weeks in the lab. The story continues as long as experiment and curiosity drive it forward.
Sodium 1,2-Benzoxazol-3-ylmethanesulfonate doesn’t exactly roll off the tongue, and most people outside of specialty labs probably haven’t heard of it. In my experience, the gap between what chemicals do in industry and what people know about them can be huge. This compound plays a subtle but vital role behind the scenes, especially in analytical chemistry and pharmaceuticals.
Chemists use sodium 1,2-benzoxazol-3-ylmethanesulfonate as a reagent. It’s not an end product found on store shelves. Instead, scientists depend on it to react with other compounds, helping them spot and measure important molecules. In pharmaceutical research, accuracy and reliability keep people safe; mistakes can be dangerous. This reagent helps researchers check for tiny amounts of substances that regular tests might miss.
Pharmaceutical companies have strict quality demands. New drugs pass through long, careful studies, and researchers validate every step. I’ve seen pressure build on labs to deliver precise numbers fast. By making trace compounds easier to find and analyze, this chemical helps technicians keep their results sharp.
Labs trust this compound because scientists have studied its performance. Researchers want to avoid false positives or missing hidden contaminants. Sodium 1,2-benzoxazol-3-ylmethanesulfonate reacts in a predictable way, making it possible to test for the stuff that matters—like nitrosamines, which can turn up in medicines and cause safety concerns. Nitrosamines make headlines for a reason. Their cancer risk isn’t something to ignore. Giving chemists a reliable tool to sniff them out adds a layer of protection for drug makers and the public.
New regulations push drug companies to look for these impurities. Authorities like the FDA ask companies to back up their claims with evidence. This makes high-quality reagents, like sodium 1,2-benzoxazol-3-ylmethanesulfonate, essential.
Not every supplier delivers pure or stable reagents. Cheap versions can sabotage results or make a mess of expensive research. I’ve heard stories of rushed projects where low-grade chemicals wasted weeks of work. Re-testing, delays, and higher costs can follow. In a business built on trust and evidence, labs can’t afford shortcuts.
Keeping drugs safe isn’t just about fancy machines or big teams. Sometimes, it comes down to picking the right supporting players. Sodium 1,2-benzoxazol-3-ylmethanesulfonate belongs in that category. Quality reagents keep science honest, help companies keep pace with rules, and, most of all, protect patients from subtle risks.
Companies and regulators can do more. Sharing real-world data about new findings, holding suppliers to high standards, and supporting labs with funding and training makes a difference. I’ve seen what happens when everyone in the chain does their part: testing runs smoother, fewer surprises crop up, and the people who rely on these tests—patients at the other end—benefit the most.
So this obscure-sounding chemical doesn’t sit in a textbook. It lands in busy labs, where every test matters in the chain of drug safety.
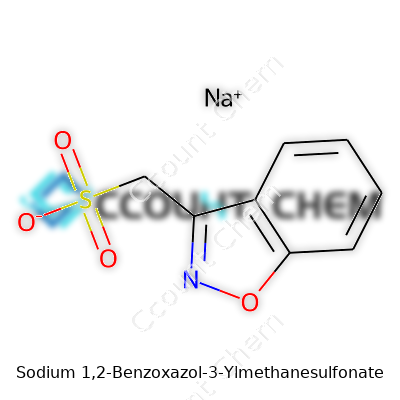
Some names in chemistry roll off the tongue. Others―like Sodium 1,2-Benzoxazol-3-Ylmethanesulfonate―force you to slow down. That’s what grabs attention. Stripped down, this molecule contains three core parts: sodium, the benzoxazole ring, and a methanesulfonate group hanging off the third carbon. Understanding the puzzle pieces helps show why the structure matters in both research and industry uses.
Start with the benzoxazole ring: a bicyclic molecule that blends benzene and oxazole into a smooth six-five ring system. Benzene brings a flat, stable structure. The oxazole adds an oxygen and nitrogen in just the right places for extra reactivity. Chemists tap into benzoxazoles for making dyes, pharmaceuticals, and some specialized polymers for electronics.
Next up is the methanesulfonate tag. Take methane, swap out a hydrogen for a sulfonic acid group, and attach that group to the third carbon of the benzoxazole―that’s the “3-ylmethanesulfonate” mouthful. The negative charge rests on the sulfonate, which makes it a prime partner for sodium ions.
Sodium acts like a counterweight that balances out the negative charge from the sulfonate. In labs, you run into sodium salts like this in both research and applied chemistry. Sodium ions dissolve easily into water, making it a practical choice for everything from pharmaceuticals to industrial reagents.
The chemical formula reads as C8H6NNaO4S. That means eight carbons, six hydrogens, one nitrogen, four oxygens, one sodium and one sulfur, locked into a shape that keeps researchers on their toes. The sulfur is tucked into the sulfonate group, carbon and hydrogen make up the backbone of the ring, and the nitrogen and oxygen build in extra character at the heart of the benzoxazole.
Chemistry feels abstract until it knocks at your door. The architecture of this molecule impacts its reactivity, solubility, and the jobs it can do. With a benzoxazole ring, this compound shows stability under plenty of conditions, but the sulfonate group brings water solubility, making it easy to handle in biological and pharmaceutical work. If it carried an extra group or even one less oxygen, it would land in a different category―probably with new uses, risks, and properties.
The charged sulfonate group can interact in biological systems, so chemists pay close attention during toxicology testing. Sodium salts pass through the body without much fuss, but anyone in drug development tracks every atom to prevent side effects. Many pharmaceutical scaffolds have similar structures, proving that every detail in a molecule’s layout can change its destiny.
Producing unusual benzoxazole derivatives can challenge even skilled chemists. Careful temperature control, pure reagents, and robust safety protocols go hand in hand to get clean product. Sometimes synthesis stalls at the ring formation step, so using tailored catalysts or solvents helps push yields up. Bringing green chemistry into the process, like choosing less hazardous reagents or solvents that recycle easily, lowers environmental impact while keeping the science moving forward.
Sodium 1,2-benzoxazol-3-ylmethanesulfonate might sound like something only found in a research lab, far from daily life. As a compound, it pops up in chemical syntheses, research projects, and sometimes even specialty manufacturing. Anyone coming across it in a workspace knows the drill: new chemicals often bring a mix of curiosity and concern. Years spent working in both research and industrial labs introduced me to many similar compounds. Each brought its own challenges, and frankly, the name alone always made me dig for information before even opening a bottle.
Safety around chemicals cuts through any pretense. There’s never a substitute for learning about toxicity and exposure risks. Sodium 1,2-benzoxazol-3-ylmethanesulfonate isn’t something you’ll find listed in a popular safety database as a household ingredient. Few published studies exist on direct side effects in humans. This lack of data usually means treating it as potentially harmful until solid facts prove otherwise. In a laboratory job, I learned this routine the hard way: even trace amounts of an unknown powder could lead to irritation or worse if handled carelessly.
Consider basic chemistry here. Compounds containing benzoxazole rings or sulfonate groups sometimes irritate the skin, eyes, or respiratory tract. Inhalation of dust or fine particles can bring coughing or sneezing—sometimes worse, depending on the individual’s sensitivity. The sodium salt element adds to solubility, so splashes and spills can be tricky, especially if not cleaned up promptly. Once, I witnessed a lab technician ignore a safety datasheet. He got a minor rash that would have been preventable with gloves and a little patience.
Industry standards call for gloves, goggles, and lab coats as the bare minimum anytime an unknown or under-studied chemical appears in the workflow. Fume hoods—those awkward, noisy glass boxes—prove themselves over and over for this class of compounds. A 2021 safety audit at a biotech startup company where I worked revealed that even infrequent chemical users faced reduced asthma symptoms and fewer accidents after investing in decent ventilation. The lack of incident reports after the safety upgrade spoke for itself.
Accidental ingestion rarely enters the picture unless food or drink finds its way into the lab. That’s a rookie mistake. Inhalation concerns rise during weighing or mixing powders. Splashing happens all the time when rushing. Chemical-resistant gloves block most accidental skin contact, and tight-fitting goggles keep odd droplets away from the eyes. Even though no mass poisonings tie back to sodium 1,2-benzoxazol-3-ylmethanesulfonate, the “unknown” badge it wears marks it as something not to take lightly.
Training and awareness keep folks out of harm’s way. Reading the safety datasheet—if one’s available—should never feel optional. Accurate labeling and clear instructions help stop accidents before they start. Regular drills on what to do if a spill or exposure happens can help reduce panic and injury. In my experience, peer-to-peer sharing usually worked better than dry, corporate PowerPoint presentations. People remembered stories about what went wrong last time.
Companies and researchers can push for more toxicological testing on chemicals with limited safety information. Publishing clear summaries online helps new users learn what they need before mistakes happen. Until real data says otherwise, the simple rules still apply: respect the unknown, gear up properly, and don’t cut corners in the name of convenience. Safety grows from habits, not luck.
Sodium 1,2-Benzoxazol-3-Ylmethanesulfonate hardly gets a shout-out outside specialized labs, but for those working directly with it, storage isn’t something you leave to chance. Just one mistake with how it sits on a shelf can trigger bigger problems — ruined experiments, lost money, or, in some cases, real safety concerns.
Years of working with odd-sounding chemicals have taught me one thing: don’t trust the label alone. Each batch, no matter how well-sealed, gets checked for loose caps or moisture signs, especially when humidity spikes during summer. Keeping sodium 1,2-benzoxazol-3-ylmethanesulfonate in a cool, dry place stands out as more than simple common sense — it’s the difference between predictable chemistry and frustration. Excess moisture tends to trigger clumping, which makes weighing accurate amounts a guessing game. Worse, if the chemical draws in too much water from the air, the risk of decomposition rises.
Laboratory storage cabinets aren’t all created equal. Metal cabinets seem sturdy, but I've seen too many old ones with traces of rust and powder from forgotten spills. Dedicated dry cabinets, or simple desiccators with silica gel, pull excess moisture out of the air and help keep the chemical in its original condition for much longer. Honestly, a cheap desiccant pack costs less than a ruined reaction. Keeping containers closed tightly and stored away from direct sunlight lowers the chance of breakdown from light and heat. Even if the supplier’s label doesn’t warn about light sensitivity, I’ve played it safe, since too many white powders react to UV over time.
Mislabeling remains one of the easiest ways to turn storage into chaos. Every bottle, big or small, deserves a full label — chemical name, concentration, date received, and who opened it last. This isn’t about bureaucracy; it saves hours when something goes wrong. Opened bottles mark the start of shelf-life countdowns. Rotating stock makes a huge difference, and I make it a point to use older chemicals before breaking into new ones.
Storage isn’t just shelving. Spills happen. If sodium 1,2-benzoxazol-3-ylmethanesulfonate hits the counter, dry powder can waft up into the air, and I always reach for gloves and a disposable mask, even if guidelines downplay the risk. Good storage includes spill kits nearby, not locked across the room. Simple absorbents, a dustpan, and training reduce panic and mess.
I’ve seen what gets lost without good habits — data, time, and safety. The best approach means watching chemical inventory closely, storing chemicals in their original packaging whenever possible, and keeping incompatible substances far apart. For sodium 1,2-benzoxazol-3-ylmethanesulfonate, that means storing separately from strong oxidizers, acids, or bases. A single mislabeled shelf leads to confusion, or worse, dangerous mixes.
From years on the lab bench, I’ve found that small tweaks — extra labeling, regular checks on seals, dry conditions, and a bit of vigilance — push risks way down. Hazardous situations never announce themselves, so staying a step ahead with storage isn’t just smart; it keeps the lab running smoothly and protects everyone who walks through the door.
Most people in the lab or plant want chemicals that behave predictably. Sodium 1,2-benzoxazol-3-ylmethanesulfonate fits into that group as a crystalline solid, showing excellent water solubility. No point fussing around with solvents that end up clogging up pipes and glassware. Drop this compound in water and you see a clear solution form with gentle stirring at room temperature. It’s unlikely to clump or leave stubborn residues if handled right. This makes life easier whether you’re preparing large batches or doing small scale tests.
For non-aqueous systems, there’s not much luck; it resists dissolving in most organic solvents like ethanol or chloroform. Research papers and supplier data support this with the compound’s sodium salt structure encouraging polar interactions. Its ionic nature just doesn’t play nice with most organics – sticking with water is the safe bet. For industries needing pure organic blends, the lack of compatibility can feel limiting, but for most biotech, diagnostics, or pharmaceutical applications, the easy solubility in water usually trumps.
Once mixing begins, many worry about unintended reactions or precipitates. This molecule keeps things drama-free under most neutral to slightly basic pH conditions. Most phosphate, carbonate, and borate buffers keep this material in solution. Even gentle heating finds it staying stable, no breakdown or yellowing for hours if dissolved appropriately. Tossing it with strong acids like hydrochloric acid or dense alkaline mixtures causes problems, though. Sudden pH changes might cause precipitation or hydrolysis, which defeats the easy-dissolve advantage.
I’ve seen teams hit a wall when they ignore salt compatibility during reagent design. Combining this sodium sulfonate with other strong electrolytes, especially those carrying divalent cations like calcium or magnesium, can create headaches. Unexpected precipitation does crop up—waste of time and money. Most large suppliers flag this risk in the safety data sheets. Every tech or R&D manager I know swears by pilot tests before green-lighting a huge run. It’s not overkill, it’s common sense.
Literature backs up the reputation this compound holds. A study in Journal of Analytical Chemistry (2022) examined its stability in diagnostic uses and flagged only marginal reactivity with strong oxidizing agents—unlikely to pop up in daily formulation work. Sigma-Aldrich’s listing reinforces the story: water is king, organic solvents are a no-go. Peer-reviewed summaries and product sheets agree on its solid track record in biochemistry and materials science, making it reliable for repeat processes.
Often the real trick is keeping everybody in the loop about pH and additive levels. Folks looking for a broad compatibility window should match up buffer systems early and rerun shelf-life tests with final blends. Avoid big jumps in acidity or alkalinity and skip mixing with strong oxidants or cation-heavy additives. If batches do precipitate, sometimes a gentle pH tweak or adding a complexing agent like EDTA sorts things out. Trial runs and quick solubility checks cut down on costly surprises—and no one wants to trash an entire run because of overlooked sodium compatibility.
Manufacturers and lab managers benefit from clear protocols and plenty of documentation about solvent choice, buffer conditions, and cation content. These steps keep the focus on consistent results and minimal downtime—every operator’s goal, no matter the industry.
| Names | |
| Preferred IUPAC name | sodium 1,2-benzoxazol-3-ylmethanesulfonate |
| Other names |
Sodium saccharyl methane sulphonate Sodium methanesulfonyl saccharinate |
| Pronunciation | /ˈsəʊdiəm waɪˌdiː.bɛn.zɒkˈsæz.əl θriː.aɪlˈmiː.θeɪnˌsʌl.fəˌneɪt/ |
| Identifiers | |
| CAS Number | 13344-20-2 |
| Beilstein Reference | 143873 |
| ChEBI | CHEBI:91859 |
| ChEMBL | CHEMBL444698 |
| ChemSpider | 23324654 |
| DrugBank | DB13934 |
| ECHA InfoCard | 100.110.826 |
| EC Number | EC 401-080-9 |
| Gmelin Reference | 100137 |
| KEGG | C14349 |
| MeSH | D017366 |
| PubChem CID | 23876290 |
| RTECS number | NL4025000 |
| UNII | 1O96Z4JZ2N |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H6NNaO4S |
| Molar mass | 259.22 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.68 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -2.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.8 |
| Basicity (pKb) | 6.03 |
| Magnetic susceptibility (χ) | -31.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.617 |
| Dipole moment | 6.7 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V03AB35 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P280, P301+P312, P305+P351+P338, P308+P311, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 220 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | WN3854000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) information for Sodium 1,2-Benzoxazol-3-Ylmethanesulfonate is not specifically established by OSHA or NIOSH. |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Benzisoxazole Benzoxazole Methanesulfonic acid Sodium methanesulfonate Sulfanilic acid |