Chemists started isolating methanesulphonic acid in the mid-19th century, figuring out ways to link organic sulphonates with rare metals. The union of silver with methanesulphonate became more relevant as industries sought new paths beyond standard silver nitrate and silver acetate. Tech industries asked for better conductors and more stable compounds. Silver methanesulphonate picked up steam in the late 20th century, especially in electroplating and electronics. Research journals from the 1980s already talk about this salt as a response to old-school cyanide-based baths, which carried health problems and tough regulations. Over time, labs learned to make it in purer and more consistent batches, which started feeding both universities’ curiosity and manufacturers’ hunger for cutting-edge, safer chemicals.
Silver methanesulphonate, known in some circles as silver mesylate, falls squarely into the family of organosulphur compounds. This white or colorless crystalline powder, soluble in water and polar aprotic solvents, often stands out due to its high purity and ease of incorporation into mixes. Its main draw comes from its role in silver plating and battery technology, providing a less hazardous alternative for companies seeking to cut down on environmental and regulatory headaches. Thin-film manufacturing and printed electronics frequently turn to it, especially as sectors push for greener tech. Looking across trade catalogs, it’s clear buyers choose this salt for better bath conductivity, smoother grain structure, and reduced downtime linked to sludge buildup.
Silver methanesulphonate comes with a molecular formula of AgO3SCH3 and a molar mass around 230.03 g/mol. At room temperature, it appears as a dry, solid material that clings easily to moisture—hence, labs recommend airtight storage. It dissolves readily in water, forming clear, stable solutions at standard concentrations used in plating and electrodeposition. Chemists appreciate its lack of strong odor or offensive fumes, unlike many older silver salts or organic sulphonates. Thermal stability allows for long shelf life under controlled conditions, and it resists decomposition at moderate heat. Heavy shaking or extended UV exposure, though, starts to break it down, releasing traces of sulphur dioxide and silver metal. Its conductivity in solution outpaces many classical silver compounds, a property that directly impacts industrial process efficiency.
High-purity forms, often above 99%, drive most commercial use of silver methanesulphonate. Common packaging includes sealed glass bottles or polymer-lined drums, with net weights tailored for lab or bulk buyers. Clear labeling, as dictated by GHS and regional chemical codes, shows the compound’s full name, molecular signature, hazard pictograms, batch number, and recommended storage temperature. Manufacturers often specify trace impurities, like sulfate or nitrate, that may affect plating or research outcomes. Conductivity, solubility at ambient and elevated temperatures, and pH range in solution usually get documented for quality assurance. Safety warnings on the label follow industry guidelines for silver compounds—risk of skin and eye irritation, need for gloves, and directions on waste disposal.
Making silver methanesulphonate involves reacting methanesulphonic acid with a silver source, most often silver oxide or silver carbonate, under strict moisture-free conditions to prevent side reactions or product loss. The reaction frees water and forms the target salt, which workers then filter and dry under vacuum. I recall sitting in a university lab, watching chemists fuss over crystal growth and filtration—the challenge always revolved around keeping out stray ions and ensuring water got drawn out without clumping or cake formation. After precipitation, the product demands thorough washing with cold solvents to remove excess acid, dried at low heat, and stored airtight. Small impurities or poor moisture control turn into headaches downstream, especially for companies producing electronic-grade material.
In chemical synthesis, silver methanesulphonate serves as a clean silver ion donor for organic transformations, especially those involving cross-coupling or ligand exchange. Coupled with its high solubility, it finds acceptance in processes like Ullmann reactions, where its ions join with aromatic halides. Replacing methanesulphonate groups with other functional groups forms an entire class of derivatives, useful in fine chemicals and specialty material production. When introduced to strong bases or reducing agents, it releases metallic silver—one reason it works well in advanced mirror-making or customizing conductive inks. Changes in temperature, solvent, or pH can trigger rapid precipitation or facilitate ion-exchange steps. Researchers push these reactions toward higher yields or selectivities, eager for clean outputs and minimal byproducts.
Beyond "silver methanesulphonate," other recognized names fill material safety sheets and spec documents: silver mesylate, silver(1+) methanesulfonate, and methylsulfonic acid silver salt. International suppliers sometimes use local translations or abbreviations, such as AgMS for purchasing departments. It’s not uncommon to find trade names attached by major chemical companies, but the core identifiers center around the sulphonate backbone and silver ion. This lexicon matters, especially during cross-border orders or customs checks, since errors trigger shipment delays or mislabeling fines. Chemists scanning databases often search using CAS Number 2386-57-4, knowing a product with a similar name could carry different impurities or physical behavior.
Working with silver methanesulphonate regularly calls for strict attention to workplace hygiene and exposure controls. Even though this compound scores lower in acute toxicity than traditional silver cyanide or nitrate, it still causes skin and eye irritation, with rare reports of asthma in oversensitive individuals. Direct inhalation or ingestion should always be avoided, as silver ions risk bioaccumulation and related argyria if mishandled. Standard lab practice involves gloves, goggles, and ventilated benches, with ready access to eyewash and spill kits. Waste solution gets collected separately from other silver streams, with recycling yards eager to reclaim dissolved silver—both for environmental reasons and the sheer value. Companies keep digital logs of air and surface monitoring, since insurance and regulators demand proof of compliance.
Industrial silver plating banks heavily on this compound thanks to its superior bath stability and fast ion transport. Electronics manufacturers trust it for thin-film and printed circuit board fabrication where trace impurities affect signal strength or longevity. its role in making high-density capacitors and advanced batteries has grown, as batteries move beyond lithium and traditional chemistries. Analytical labs use silver methanesulphonate for titrations and ion-selective electrode manufacture. Artists and craftspeople exploring new types of conductive paints have tested it for layering on glass and ceramics. Each sector values the headset of features: easy dissolution, low-tox process byproducts, and compatibility with existing hardware. Green chemistry fans point out its lower risk compared to traditional silver sources.
New research looks into using silver methanesulphonate for nanoparticle synthesis and custom conductive coatings, especially where size and shape play a role in function—think sensors or medical implants. Teams experiment with swapping in organic modifiers to tune solubility or reactivity, targeting performance improvements in batteries and supercapacitors. Some labs try immobilizing the compound on polymer supports for reusable catalytic beds. Others seek blends with ionic liquids to unlock higher thermal stabilities for use in power electronics. Funding for this research comes from both industry partners eager for high-throughput scale-up and public agencies backing safer alternatives to outdated, toxic silver supplies. Research networks foster international collaboration, with preprints and patents showing steady year-on-year growth.
Toxicologists keep a steady watch on the biocompatibility of silver methanesulphonate, since silver’s accumulation and persistence present special challenges. Laboratory animals exposed to high doses show mild to moderate tissue effects, mostly tied to the silver, not the methanesulphonate. Test tube results show low cytotoxicity at practical industrial concentrations. Chronic exposure data remains limited, driving more long-term studies around waste management and environmental spill scenarios. Researchers in occupational health examine skin and respiratory uptake, collecting case studies from manufacturers and plating shops. Water discharge rules mandate strict filtration and reclamation, since regulators want no repeat of heavy metal pollution from past decades. Public datasets on silver compounds provide a yardstick, but ongoing studies continually shape safety advice and workplace protocols.
Industry’s shift toward green chemistry and sustainable sourcing nudges silver methanesulphonate into an expanding field of use, covering energy storage, flexible electronics, and advanced catalysis. Startups optimize preparation for cleaner, high-yield runs, exploring cheaper silver recovery from spent electronics. Projected demand for electric vehicles and 5G/6G infrastructure sparks new applications, especially where conductive pastes must combine performance with sustainability. Regulatory trends favor non-cyanide, non-nitrate baths, so plating houses plan to swap over systems and retrain workers. Labs test hybrid organic-inorganic frameworks, eyeing medical devices that require silver-doped layers for antimicrobial properties. Venture funds flow toward technologies that can safely, cleanly, and cost-effectively deploy silver salts at scale, and research communities publish an increasing number of open-access papers, hoping to meet tomorrow’s challenges before they arrive.
Anyone who’s worked in electronics or advanced manufacturing will know the puzzle of finding reliable materials for specific tasks. Silver methanesulphonate, with its tongue-twisting name, quietly supports some important work across several industries. This compound delivers a stable, water-soluble source of silver ions, which matters in any setting where moving beyond traditional silver nitrate or silver cyanide offers both safety and performance gains.
Years spent around circuit board fabrication lines have convinced me that the chemistry behind metal deposition deserves more attention. In the mid-2000s, moving away from cyanide-based processes felt urgent. Silver methanesulphonate allowed engineers to plate silver without relying on the hazards of cyanide baths. The compound helps lay down a shiny, highly conductive metallic layer on printed circuit boards, essential for signal flow in today’s crowded microelectronics.
As demand surged for smaller, tightly-packed circuits, uniform silver coating became critical. The ion exchange properties of this compound enable clean, reliable deposition at lower toxicity levels. Workers handle fewer dangerous fumes, and waste disposal grows less complicated. Anyone who’s ever toured a production floor will recognize the value of reducing hazardous exposures.
Jewelry makers and decorative platers run into similar problems. Traditional silver salts involved in plating can leak harmful substances into water streams, especially from small operations without industrial wastewater treatment. Silver methanesulphonate, due to its greater solubility and lower risk profile, shows up in eco-conscious plating shops. By offering a less-toxic bath, this compound helps producers maintain bright, strong plating that stands up to daily wear.
Cheaper alternatives often struggle with tarnishing, rapid degradation, or supply inconsistency. The industry quietly benefits from the high purity levels and reduced faulty runs that silver methanesulphonate supports. Shifting the recipe can mean achieving cost savings in maintenance, all while standing just a step closer to environmental compliance.
Energy researchers inching solar cells toward better efficiency also turn to this compound. Silver’s conductivity suits the fine contacts required in solar panels, while traditional silver sources struggle with residue or messy waste streams. Silver methanesulphonate’s clean dissolution makes precision deposition straightforward. Slight tweaks in the wet chemistry along the panel’s surface can shave losses and help panels reach the grid faster.
Reliable renewable energy depends on clean materials from start to finish. New solar outfits in Asia and Europe report smoother surface finishes and longer device lifetimes where they swapped their old compounds out. Every incremental improvement in these panels pushes the world that much closer to greater energy independence.
For companies looking to upgrade or diversify their materials, switching over isn’t always simple. Sourcing high-quality silver methanesulphonate and retooling baths cost money up front. Training staff and shifting safety protocols can meet internal skepticism. Still, with regulations around toxic chemicals tightening across many countries, this compound starts making a lot of sense for anyone who wants long-term viability.
Silver methanesulphonate stands as a clear example: a material that doesn’t grab headlines, but supports valuable changes—better safety, cleaner water, and stronger results for manufacturing at nearly every scale.
Anybody who’s spent time in a chemistry lab knows silver doesn’t just shine on jewelry. Its compounds, especially Silver Methanesulphonate, offer plenty to chemists and inventors. The chemical formula for Silver Methanesulphonate is Ag(CH3SO3). You get a single silver ion paired up with a methanesulphonate group. Silver brings one positive charge, the methanesulphonate anion balances with a single negative charge.
Chemists like myself have seen how Silver Methanesulphonate steps in as an electrolyte for silver plating. In the trenches of industry, traditional silver salts often come bundled with cyanides and heavy metals regulators dread. Methanesulphonate helps to kick those toxins off the table. The formula might look simple, but the reaction it allows is anything but: Ag+ combines with CH3SO3- to help deposit pure metallic silver.
The move to Silver Methanesulphonate is more than a chemical swap. It shows up wherever there’s a push to lower workplace hazards. Methanesulphonates break down more gently if a spill or leaky tank enters the picture. The molecular structure merits a second look: one silver atom, a small organic chain, and a sulfonate group. This combination brings surprising solubility, which is a big plus for industries chasing “greener” processes.
Facts back this up. Traditional cyanide-based silver baths have caused plenty of accidents over the decades. Poisoning risks forced many companies to rethink processes as regulations started pinching. Swapping in Silver Methanesulphonate drops that risk almost overnight. The Environmental Protection Agency in the United States lists methanesulphonates as low-toxicity compounds—and my experience lines up with that. Fewer headaches over storage, fewer emergency drills, and more peace of mind.
A big hurdle today ties back to cost and connection. Silver isn’t cheap and demand just keeps rising because of electronics, medicine, and solar tech. Methanesulphonate-based routes use silver more efficiently, pulling better yields per ounce bought. Still, it isn’t magic—there are limits. Price spikes in silver can slow the green transition, even with a safer formula on hand.
I’ve watched suppliers in the chemical trade try to bridge that gap. Some recycle more silver out of used electrolytes. Others form partnerships, rounding up methanesulphonic acid made from natural gas or methane. Local production cuts shipping costs and lowers greenhouse gas numbers. If governments keep up their push for cleaner plating lines, expect to see Silver Methanesulphonate popping up far beyond specialty labs.
Education helps, too. Chemical engineering students read about these formulas but rarely see the impact until their first job. More universities now run pilot projects using Silver Methanesulphonate baths, giving students hands-on time with safer reagents. This shift in training reduces lab accidents and shapes a new kind of workplace—one that values both human health and high precision.
Chemicals don’t shape the world by formula alone. They earn their place by what they make possible—and what they help avoid. In a future aiming for better safety, Silver Methanesulphonate deserves its spot on the workbench and production floor.
Silver methanesulphonate shows up in several labs, especially in electronics and plating. Most people figuring out how to handle it quickly realize its quirks. The key isn’t just having a label or looking up the latest spec sheet. My early chemistry years taught me that storage choices are not simply a box-checking exercise—ignoring the real hazards can impact both health and a project’s bottom line.
Nobody wants their chemical stash to turn into a science experiment gone wrong. Moisture is the enemy. Silver methanesulphonate pulls in water from the air, which messes with purity and can form clumps that ruin weigh-outs. I’ve watched careless storage turn a crisp white powder into damp chunks in less than a week. A tightly sealed container, made of polyethylene or glass, keeps air and water out. Placing that container in a cool, dry cupboard far from windows or vents makes a world of difference.
Direct sunlight speeds up the breakdown of countless lab chemicals. Silver methanesulphonate isn’t immune. I’ve seen products fade and lose quality from hours spent on sunny shelves. Using opaque or amber containers, and keeping supplies in low-light storage, preserves stability. On top of that, nearby reactive substances pose a risk—mixing acids or strong bases, even by accident, can spark unwanted reactions. A dedicated storage area reserved only for relatively low-reactivity salts helps prevent these mistakes.
Cross-contamination remains a real threat in busy labs. Scooping from multiple bottles with the same spatula leaves unknowns in every container. Double-dipping along with careless handling sends purists into a spin and can ruin sensitive processes, such as precision plating or microelectronics. Clean tools every time, and label each item clearly. I always push for training—even short reminders work better than trusting luck or hoping someone “should know better.”
Regulations for chemical storage evolved for a good reason—dodging trouble. Most lab managers follow local and international rules to avoid legal and physical risks. Proper labeling, inventory sheets, and updated Material Safety Data Sheets save more than paperwork headaches. Quick access to spill kits, gloves, and eye protection means fewer panicked moments if something tips or tears. Little steps, like storing only what’s needed and keeping a disposal plan ready, prevent stockpiles from becoming hazards.
Over-ordering leads to full shelves and extra risk. My best storage outcomes happened in places where people respected quantities: small, manageable amounts, not bulk buckets sitting for years. Keeping only what’s necessary means chemicals get used while still fresh, and waste stays under control.
Bottom line: handling silver methanesulphonate responsibly means more than just reading a label. Dry containers, cool storage, attention to contamination, respect for safety rules, and smart quantities each play a role. Good storage wins every time over taking chances and hoping for the best.Silver methanesulphonate rarely finds its name in chemistry textbooks outside industrial circles. The chemical’s unique properties catch the eye of manufacturers, especially those working with precious metal plating in electronics. Yet every material, no matter how niche, deserves honest scrutiny for safety.
On paper, this silver salt sits in a modest spot—it isn’t as notorious as mercury or as dramatic as sodium cyanide. Safety data varies, with some research documents suggesting low toxicity in small amounts, especially compared to other silver compounds. Still, absence of dramatic hazard labels does not mean users can breathe easy and toss all caution out the window.
Silver compounds, in general, bring their own baggage. Chronic exposure to silver dust or salts can cause argyria, a bluish-gray discoloration of the skin and eyes. That’s not something any worker wants, even if the condition looks harmless compared to acute chemical poisonings. I’ve walked through plating shops and watched how fine powders get spread around, almost invisible to the eye, and wondered how much ends up in lungs and on skin weeks or months later.
Despite its moderate profile, silver methanesulphonate can still irritate the eyes, skin, and respiratory tract. Spills and dust aren’t just paperwork—they’re health issues. Chemical burns from silver salts may seem rare, but anyone who has watched silver nitrate stain a fingertip knows the stuff lingers. Inhalation risk grows as particle sizes shrink. Short-term exposure might not trigger panic, but repeated contact slowly stacks up. Hardly any factory worker goes home and brags about not wearing gloves—the price of overconfidence shows itself later.
Waterways can't deal with heavy metals. Even low concentrations from rinsing solutions end up in municipal systems, and silver is toxic to fish and aquatic life. Environmental fate gets harder to track with organosulfonates, since these groups can sometimes pass through standard water treatment.
Practices in handling often outpace the law. Written exposure limits for silver itself exist, but methanesulphonate-specific limits rarely surface in regulations. That should never excuse a lack of safety. Closed systems stop powders from floating. Fume hoods keep air clear. Occasional spot checks with personal monitoring can flag the early signs of trouble before a workplace turns into a test case.
Simple fixes work. Disposable gloves and lab coats cost little compared to cleanup and lost time. Using forced-air ventilation, collecting spills right away, and treating rinses with precipitation agents keeps silver out of both lungs and rivers. Some companies have started running waste streams through small-scale ion changers to catch silver before it goes anywhere else. Watching a company take the step to reuse or recycle silver instead of discharging waste always seems smarter than risking a visit from environmental inspectors—or having to face local fishermen.
Training makes all the difference. Too often, veteran workers ignore new hazards, assuming everything’s as safe as yesterday’s setup. Sharing information—openly, and with clear real world consequences—gives everyone in the shop a fighting chance at a safer shift. I’ve seen one frustrated worker quit after a skin reaction, only to retrain in the next plant, this time with gloves on from day one.
No chemistry shop or electroplating line can afford to overlook the cumulative effects of silver salts like methanesulphonate. It won’t explode or knock you out in minutes. The risks add up slowly, in dust, grime, and rinsewater. Honest talk and practical habits, more than regulations alone, protect people and planet from chemicals whose dangers don’t always shout from the label.
Silver methanesulphonate draws the eye. To a chemist, it usually means a small bottle filled with a white powder or crystalline mass. If you’ve spent time in a synthesis lab, you recognize its bright, almost sparkling look—no odd hues, no suspicious specks. This pure appearance matters more than just for show. Impurities often show up as grayish tinges, clumps, or sticky residue. That’s not what anyone wants during a reaction, especially if you’re working on delicate electronics, plating processes, or pharmaceutical products where contaminants can ruin results.
Companies and scientists want silver methanesulphonate that doesn’t just look clean, but tests clean. Chemistry works on precision. A silver content of 99.5% or greater matches the demands of electronics or surface treatment. High purity means fewer side reactions, higher yields, and consistent performance in every batch. To put it plainly, labs can’t afford to gamble with products where every atom counts. Lower purity versions may carry trace metals, moisture, or organics that alter product quality. Reliable suppliers publish real data, not vague promises: certificates of analysis show total purity, trace element profiles, and sometimes even water content by Karl Fischer titration.
Digging into past lab work, a tell-tale sign of good silver methanesulphonate is how easily it dissolves in water or methanol. No gritty residue collecting at the bottom. A single moisture exposure can make an otherwise free-flowing powder form sticky clumps, harming reactivity and accurate weighing. Proper storage plays a role—tightly sealed amber glass bottles, cool cupboards, desiccators for long-term keeps. Some companies skirt around quality controls by bottling in cheap plastic or offering “off-white” substitutes. These short-cuts end up wasting valuable time and money trying to clean up the chemistry.
Good manufacturing practice requires more than just a clean production line. Rigorous purification such as recrystallization, precise solvent handling, and well-maintained equipment keep contamination low. Testing batches by techniques like atomic absorption spectroscopy or ICP-MS exposes even trace contaminants. Each step costs extra effort but pays off for those relying on the end product. Routine audits and supplier spot checks keep the system honest. Sourcing from organizations that take calibration and validity of testing seriously really pays off. The few dollars saved with bargain supplies turn into thousands lost by failed reactions or defective devices.
Judging a chemical by looks alone is risky, though not pointless. While bright, white crystals often indicate high purity, only data from a trusted lab can confirm the real numbers. Responsible suppliers, experienced chemists, and sharp-eyed buyers share the same goal: chemicals that look right, test right, and deliver every single time. Real-world lab success starts here, not just with pretty packaging.
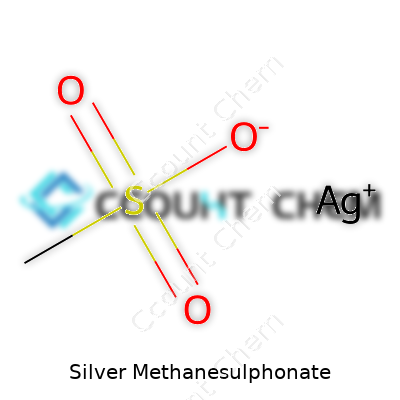
| Names | |
| Preferred IUPAC name | Silver methanesulfonate |
| Other names |
Silver(I) methanesulfonate Methanesulfonic acid silver(I) salt Silver mesylate |
| Pronunciation | /ˈsɪl.vər mɛˌθeɪn.sʌlˈfəʊ.neɪt/ |
| Identifiers | |
| CAS Number | [2924-65-6] |
| Beilstein Reference | 3443964 |
| ChEBI | CHEBI:86365 |
| ChEMBL | CHEMBL3356432 |
| ChemSpider | 851181 |
| DrugBank | DB11077 |
| ECHA InfoCard | 100.028.870 |
| EC Number | 401-610-8 |
| Gmelin Reference | 77127 |
| KEGG | C18635 |
| MeSH | D017580 |
| PubChem CID | 16211056 |
| RTECS number | GV8650000 |
| UNII | 2WI1539RTC |
| UN number | UN3434 |
| Properties | |
| Chemical formula | AgCH3SO3 |
| Molar mass | 186.04 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 2.48 g/cm³ |
| Solubility in water | Soluble |
| log P | -0.384 |
| Acidity (pKa) | 1.8 |
| Basicity (pKb) | 11.3 |
| Magnetic susceptibility (χ) | −24.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.480 |
| Viscosity | 25 mPa·s (20 °C) |
| Dipole moment | 4.10 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 189.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -384.5 kJ/mol |
| Pharmacology | |
| ATC code | V03AX17 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H332 Harmful if swallowed or if inhaled. |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313, P362+P364 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 250 g/L |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Silver acetate Silver nitrate Silver sulfate Methanesulfonic acid Sodium methanesulfonate Potassium methanesulfonate |