Salicylsulfonic acid has lived many lives since researchers first isolated it in the late 19th century. Chemists didn’t tap its true analytical power until the dawn of modern colorimetric analysis. Early notebooks from German and British labs reveal pages covered with calculations and sketches of glassware, signifying its rise in both qualitative and quantitative protein determination. In my own lab days, I often reached for this reagent when precision was paramount. These practical roots gave it a respectable place in analytical chemistry, but also tethered it to the responsibility of clear labeling and reliable sourcing.
Salicylsulfonic acid usually arrives as a white to slightly yellowish crystalline solid, dissolving easily in water to form a colorless or faintly yellow solution. The compound, often called 5-sulfosalicylic acid or SSA, belongs to the family of sulfonated aromatic acids. Manufacturers ship it as a reagent-grade substance, clearly indicating protein precipitation and urinalysis as key applications. On shelves, it rarely stays put for long—clinical labs and research benches always seem to run low, especially considering its shelf-stable nature at room temperature when kept dry and tightly sealed. Labels leave little to interpretation: chemical formula C7H6O6S, molar mass of 234.19 g/mol, and purity standards from 98% upwards for research use.
This solid looks deceptively plain, but it brings notable acidity and robust solubility. Melting begins around 120–125°C, paired with a notably high acidity, pKa near 1.6, thanks to its sulfonic group. Water welcomes it without fuss, and it can withstand mild heating during sample prep. The structure—the fusion of benzoic acid and sulfonic acid in a single molecule—drives both its effectiveness in protein precipitation and its limiting volatility. In the lab, handling it feels familiar: there’s never an aggressive odor, and it remains stable under standard indoor light. Chemically, it resists spontaneous change in storage, sidestepping hydrolysis and decomposition except under strong base or sustained high heat.
Bottles typically bear graphics of laboratory glassware and hazard labels outlined in red. They spell out batch number, lot-specific purity, country of origin, and expiration date. MSDS sheets stay a reach away, detailing the risk of eye and skin irritation, but confirming it stands clear of high systemic toxicity at normal concentrations. Technical data provides insight into granularity, any particulate residues, and even UV-Vis absorption peaks used for purity checks. Bar codes now replace stamped numbers, supporting traceability from plant to pipette.
Most choose the direct sulfonation route, letting fuming sulfuric acid do the heavy lifting on salicylic acid under controlled conditions—typically below 100°C to dodge over-sulfonation. I remember guiding students through the slow addition, always emphasizing that rapid temperature rise could char the whole flask before anyone noticed. Washing the product with distilled water and carefully neutralizing with sodium carbonate yields a pure precipitate. Each run leaves a strong analogy to bread baking—attention to temperature, patience for the final ‘rise’, and cleaning up the aftermath matter just as much as the recipe.
Chemists like to tweak. The carboxyl and sulfonic groups give room for derivatizations: esters for modified solubility or coupling agents for advanced analytical techniques. The acid interacts vigorously with basic proteins, one reason why it shines in clinical urinalysis as a straightforward protein precipitation agent. Slight modifications swing the molecule’s profile from water-soluble to sparingly soluble, and additions at the aromatic ring open new detection pathways for complex samples. Reactions with alkali transform the molecule into disodium or trisodium salts, which change buffering capacities—often an unsung hero for downstream analysis.
Salicylsulfonic acid also wears the labels 5-SSA, SSA, and 5-sulfosalicylic acid, sometimes appearing as sulfosalicylic acid in catalogs. Synonym confusion trips up more than a few suppliers, so it pays to verify CAS number 5965-83-3 before procurement. Each marketplace seems to have its own pronunciation and abbreviation—SSA for Europe, sulfosalicylic acid for most North American medical labs. Regardless of tag, the function remains unmistakable and the molecular signature recognizable to any chemist scanning a reagent list.
Labs often center their safety onboarding around real-world hazards rather than just regulatory boxes. Salicylsulfonic acid brings moderate risk—dust can trigger respiratory irritation, and accidental splashes often cause discomfort in eyes or minor dermatitis. Proper handling starts with basic protective gear: gloves, goggles, and solid lab practice. Emergency protocols lean on accessible eyewash stations and chemical spill kits. In facilities I worked with, periodic drills forced everyone to remember: don’t cut corners, even if the material feels less menacing than a volatile organic. Strict adherence to GHS labeling and national workplace safety laws underscore the seriousness, keeping workplace accidents rare but never impossible.
Clinical labs and analytical chemistry circles hold salicylsulfonic acid close due to its knack for precipitating proteins cleanly. Hospitals rely heavily on its accuracy in spot urine protein assays, often flagging early signs of kidney disease or monitoring ongoing disorders. Protein quantitation in research samples gets a boost from SSA’s selectivity—there’s lower background noise compared to phosphotungstic or trichloroacetic acid. Some sectors in textile and dye chemistry draw on its sulfonic group for specialty reactions and pH adjustments. Teachers introduce undergraduates to this acid for classic “unknown protein” lab exercises, appreciating both the low cost and high reliability. In environmental analysis, its ion-exchange characteristics help separate trace metals from organic matter before further instrumentation.
Modern analytical chemistry doesn’t accept standing still. Researchers test fresh applications for SSA in proteomics workflows, expanding its reach to include protein fingerprinting and post-translational modification mapping. The flexibility in forming both hydrophilic and amphiphilic pairs keeps it relevant for developing innovative separation protocols, especially with miniaturized lab-on-a-chip systems. Discussions with industry insiders reveal a drive towards greener synthesis—using less corrosive sulfonating agents and reclaiming waste streams for secondary use. Every R&D unit I’ve visited seems to have an ongoing efficiency or sustainability project targeting this classic compound, trying to balance tradition against modern demands.
Toxicity profiles for salicylsulfonic acid cover both acute laboratory incidents and long-term exposure scenarios. Though not highly toxic, research documents mild gastrointestinal upset or dermatitis at high doses or through occupational mishandling. Most clinical reviews indicate that animal testing returns low chronic impact at exposure levels well above laboratory norms, assigning it a “moderate hazard” class primarily for skin and mucosal irritation, not organ-level toxicity. Labs investing in high-throughput screens for safer reagents often use SSA exposures as a lower-bound reference point. My own conversations with occupational health officers stress routine screening for cumulative low-level exposure, especially in educational settings.
Today’s chemical industry takes an old hand like salicylsulfonic acid and asks, “How can we do better?” The move toward higher purity standards, micro-scale synthesis, and greener production lines offers a promising path. High-sensitivity analytical methods, including next-generation protein diagnostics, continue to propel demand. Chemists and clinicians anticipate broader roles for tailored derivatives in complex biological assays, while regulatory authorities push for deeper safety and environmental impact data. The pace of change means suppliers develop new grades for biopharmaceutical manufacturing, improve tracking from plant to pipette, and invest in long-term toxicity studies. Listening to feedback from both bench scientists and production engineers, the future shape of salicylsulfonic acid hinges on collaboration, squarely focused on both reliability and responsibility.
I remember my days working in a small clinical diagnostics lab. There was one reagent I kept reaching for, especially during protein tests—salicylsulfonic acid. For folks who spend hours with test tubes, this compound gets some respectful nods. It packs an ability to precipitate proteins, making it a steady choice in urine protein tests. Add a few drops to a sample, and proteins drop out, clouding up the solution. It turns what could be a guessing game into clear, measurable results.
Detecting proteins early in urine brings crucial information about kidney health. Doctors look for proteinuria to spot issues like diabetic nephropathy or early kidney disease. With salicylsulfonic acid, you get a fast, dependable reaction. Unlike some old school methods that miss smaller proteins, this one catches more types. Doctors and lab techs stick with it because it speeds up patient care and picks up subtle changes others might overlook.
Outside the hospital walls, salicylsulfonic acid does more behind the scenes. Chemists use it to analyze amino acids, purify proteins, and calibrate instruments for research. When labs set up colorimetric assays, it provides a clear, stable reaction—something much appreciated when accuracy matters. In the pharmaceutical industry, I’ve seen it used for quality control. Manufacturers want to spot impurities or breakdown products in medicines, so they turn to this acid for its reliability.
The stuff demands respect. Like other strong acids, it causes burns and irritation. Gloves and goggles prevent painful mistakes. Labs prepare solutions in fume hoods. In poorly ventilated areas, fumes trigger coughing fits or eye stinging. Emergency showers and eyewash stations always sit close by. Proper training helps, but I’ve seen complacent workers end up with ruined shirts and red eyes. The lesson sticks: don’t rush the safety steps.
Disposal brings another test of responsibility. Pouring spent solutions down the drain isn’t an option. Salicylsulfonic acid reacts with metals and living matter, so waste streams need treatment. Labs collect it in special containers, sending it to licensed hazardous waste handlers. This process costs money and time, but the alternative means pollution—fish kills in nearby streams, damage to municipal water plants, and a legal mess no one wants. Investing in proper waste management shows respect for neighbors and future generations. There’s always room for research into greener alternatives, but as of now, anyone using salicylsulfonic acid adopts the burden of safe disposal.
Technology moves forward, but old reliable reagents like this acid remain in wide use. Medical labs need fast, trustworthy results, and many commercial test kits still include salicylsulfonic acid for protein checks. Some hospitals now invest in automated instruments using different chemistries, but cost pressures keep the old methods relevant, especially where budgets run tight. Improvements in environmental safety, worker training, and detection technology may soften the rough edges, but for now, salicylsulfonic acid holds a place at the center of routine analysis and diagnostics. It’s a reminder that science thrives on simple tools—used wisely and handled with care.
Salicylsulfonic acid grabs attention in school and industry labs thanks to its protein-precipitating ability. That comes with a catch—acid burns, strong fumes, and chemical spills can happen. It only takes one mistake, like skipping gloves or ignoring fume hood rules, for a regular labeling job to land someone in the urgent care with acid burns.
For anyone working around lab chemicals, respect for the bottle rules isn’t just a formality. One errant drop will eat through organic matter—think skin or eyes—fast. Swallow or breathe in the vapors, and they hit tissue harder than a soda on an empty stomach. Over the years, stories of chemical injuries have all started the same way: a shortcut, a moment of inattention, someone not double-checking a seal. It’s not about fear-mongering; it’s about understanding real risk.
No one in a lab enjoys putting on splash goggles—they fog, they're uncomfortable—but chemical eye injuries stick for life. I’ve seen chemical burns that took months to heal because goggles sat on the bench instead of on someone’s head. Full-length lab coats matter just as much, because an apron alone won’t stop a splash from finding your wrist. Proper-fitting nitrile gloves let you grip glassware without worrying about leaks. As far as shoes go, forget sandals. Each step in a lab should promise if something spills, your feet are safe.
You don’t always smell acid fumes before they cause problems. Anyone who’s coughed through a poorly ventilated afternoon knows how a forgotten fume hood can turn a simple job into a headache that stretches into the next day. Always open containers or mix solutions in a fume hood. It’s tempting to skip this step on a busy day, but nothing beforehand fixes a burned throat.
Instruction books and safety data sheets only go so far. Real training—where supervisors walk you through spills or splashes, and you know the feel of an emergency shower handle—builds the habits that keep people whole. At one workplace, yearly refreshers included mock incidents, and people took them way more seriously after seeing just how fast an accident can unfold. Those simulations stuck better than any lecture.
Spills happen—sometimes on skin, sometimes on bench tops. Quick action stops minor problems from growing. In the case of skin contact, flushing with water right away limits burns. Not ten seconds from now, not after finding a supervisor, but instantly. Emergency eyewashes have to be ready, not blocked by boxes or tubing. Any contaminated clothing should come off fast, and, yes, that might mean losing a favorite shirt. Afterward, reporting what happened makes it possible to stop the next incident from finding someone else.
Never stack strong acids with solvents, or leave containers loose on a shelf. Leak-proof bottles, labeled and locked away, make all the difference. The right chemical fridge can keep fumes and heat at bay. In my experience, the labs I trusted most kept things simple—everything labeled, separated, and with dated inventories.
Fume hoods that actually work—inspected, clean, and with filters checked—cut down risk. Shared reminders at the start of a shift, even just going over the basics, refresh muscle memory. Automatic spill kits and neutralizers in easy reach mean small mistakes don’t spiral. Encouraging clear reporting, even for near-misses, makes it more likely that the lab learns from every close call instead of repeating it. Safety culture builds itself one good habit at a time.
Anyone who’s opened a package of chemical supplies knows the way storage shapes both safety and usefulness. Salicylsulfonic acid is no exception. If exposed to the wrong environment, it can cause headaches for lab workers and headaches for anyone who later relies on samples not kept correctly. Small mistakes—like letting it soak up moisture or stowing it near strong oxidizers—can fuel problems in the lab. And as someone who’s worked with acidic reagents myself, I’ve seen how sloppy storage erases all the hard work done to maintain consistency and keep hazards under control.
Anyone handling salicylsulfonic acid knows about its risks. This white crystalline powder can irritate skin or eyes on contact. Add water and it dissolves fast, which makes it easy to spill or splash. Fumes sting the nose, too. Even a few grams out in the open will pull in moisture from the air, turning the powder clumpy and crumbling its shelf life. I’ve seen a jar left open over the weekend, and on Monday it was a sticky mess, impossible to measure accurately.
According to the safety data sheets, salicylsulfonic acid reacts with strong bases and oxidizers. That tells me it should never go near cleaning solutions, bleach, or any unknown bottles—common sense in the lab but often ignored during busy stretches. Keeping incompatible chemicals apart isn’t just a rule for the handbook; it dodges dangerous reactions that can harm people.
The best way to keep salicylsulfonic acid safe involves simple steps. Place the powder in a tightly sealed glass or plastic container. Ordinary plastic bags won’t cut it. Choose containers that stay airtight, since exposure to humidity leads to clumping and contamination. Pick a storage area that stays cool and dark, as heat invites decomposition and sunlight speeds up deterioration. On a shelf far from any heat source or bright light, I’ve noticed the powder stays dry for much longer.
Lock away acids like this in a dedicated chemical storage cabinet, apart from bases, solvents, and anything that could trigger a reaction. These cabinets don’t just protect the chemical—they alert everyone nearby that the shelf inside isn’t for snacks or spare wires. Most science classrooms and research labs install exhaust fans around storage cabinets to keep the air clean, but even a simple, well-marked drawer works better than a crowded, catch-all cupboard.
Clear labels help. Writing the chemical name, date received, and the date you opened the container right on the label can spare future confusion and prevent accidental misuse, especially when containers look similar. I keep a habit of recording every time a jar gets opened. It sounds tedious but pays off—once, it helped us catch a batch that had started to degrade after sitting untouched for months.
Beyond storage conditions, training matters most. Every staff member or student who works in the lab must learn the risks and the rules. Regular reminders—like safety talks or posted checklists—cut down on accidents. I’ve worked in labs where everyone had to sign off after reading storage protocols, and the care people took after that improved overnight.
Looking for ways to boost safety isn’t just about rules or checklists; it shapes the trust people feel handling materials like salicylsulfonic acid. Following strong storage practices protects health, keeps experiments on track, and stops waste. It doesn’t take high-tech solutions, just a watchful eye and the willingness to respect the risks in front of us.
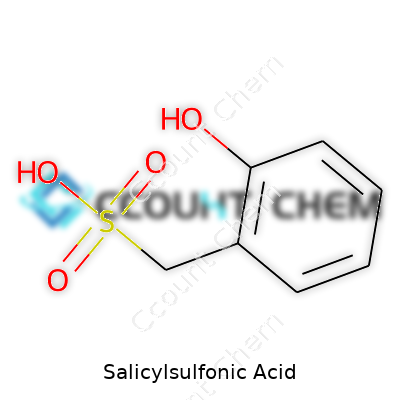
Salicylsulfonic acid might not command the spotlight like aspirin or vitamin C, but it shapes research labs and diagnostic clinics all the same. Many in science bump into its name when handling protein analysis tests or running clinical samples. People tend to seek the formula for a very practical reason: solutions and concentrations need a precise target. For the record, salicylsulfonic acid’s chemical formula is C7H6O4S. It has a benzene ring with a sulfonic acid and a hydroxy group attached, landing it squarely within aromatic sulfonic acids.
Countless undergraduate students, lab technicians, and curious researchers have stories about hunting for quick, clean ways to “crash out” proteins from solutions. Salicylsulfonic acid answers the call for protein precipitation thanks to its dual punch: the sulfonic acid makes the molecule acidic and strong at grabbing proteins out of water, and the hydroxy group adds specificity, connecting to amino groups on the proteins.
In practice, labs dissolve salicylsulfonic acid in water, adjusting concentrations depending on whether they’re clearing up a urine sample, prepping proteins for a Lowry assay, or chasing down a mysterious contaminant. No need for fancy conditions—just dissolve and go. Simpler than trichloroacetic acid and less hazardous than perchloric acid, it earns its spot on reagent shelves.
Some might gloss over molecular formulas, thinking they only matter to chemists. That strategy can backfire fast in real lab work or regulatory paperwork. Each atom in C7H6O4S guides how you store, handle, and dispose of the material. Sulfur stands out—sulfonic acids bring serious acidity and reactivity, which gives salicylsulfonic acid both its value and its safety flags.
Health and environmental considerations come front and center. Labs worldwide lean into guidelines for aromatic sulfonic acids, avoiding skin contact and keeping spills off surfaces. Disposal rules spring straight from that “S” in the formula, flagging stricter waste protocols to protect local soils and waterways. Realistically, no chemist or technician can stay hands-off from knowing what substances contain. Understanding the formula removes the guesswork and helps dodge safety slipups.
Labs running protein tests every week learn to measure enough salicylsulfonic acid to avoid waste. Bulk suppliers have pushed for better packaging, offering pre-measured packets and sealed ampules, limiting air and humidity exposure. Thinking back to long afternoons with a balance and a powder scoop, I can recall humidity clumping the powder, making dosing a headache. A precise molecular formula helps predict how the powder reacts to moisture and weighs out on a balance.
Staying grounded in the correct formula matters for research transparency, lab audits, and even medical device approval. False moves—a subpar batch or a simple typo in documentation—mean invalid results and lost confidence. Getting to the right information starts with structural clarity. The story of salicylsulfonic acid (C7H6O4S) reminds us: fine details drive scientific trust and real-world progress.
Salicylsulfonic acid pops up in plenty of chemistry labs. Whether it’s sitting in a bottle on a shelf or mixed up with other compounds, people usually want to know right away if it will play nicely with water. Water solubility matters. If a compound won’t dissolve, it gets tricky to use for solutions or tests. If it dissolves with no fuss, results generally get more reliable, and keeping lab glassware clean turns into less of a hassle. I remember more than once, holding a bottle of powdered salicylsulfonic acid and watching it vanish into water within a minute or two—it’s a straightforward process.
Salicylsulfonic acid stands out for being highly soluble in water. Its molecular structure—complete with both sulfonic and carboxylic acid groups—almost begs for water molecules to surround it. The -SO3H and -COOH groups interact with water’s hydrogen bonds, letting the compound melt right into the solution. We can actually find old and new reference books that confirm this fact—look for Merck Index entries or Sigma-Aldrich safety data sheets. Beyond anecdotes, these references make it clear: stick 5g of salicylsulfonic acid in 100 mL of water, and you’ll get a clear solution. That matters especially when you’re whipping up test reagents or buffers for biochemistry work.
Solubility has skin in the game for folks running clinical tests. Salicylsulfonic acid shows up as a key ingredient for detecting protein in urine, which helps doctors pinpoint kidney issues early. If this chemical clumped up or settled out, those tests would end up useless. You pour it in, let it mix, and a cloudy result signals the presence of protein—no cloud, no problem. All this depends on the acid being able to mingle right with water, without drama.
In my work with undergraduates, this property saved us plenty of time. Try running the same test with something that won’t dissolve, and students get frustrated with unclear readings. I remember a day one student accidentally used a bottle of a poorly labeled acid. The difference was night and day. The experiment failed, not because of technique, but because the compound didn’t dissolve—which spoke volumes about why sticking to water-soluble reagents is more than a box-ticking exercise.
Plenty of researchers encounter solubility issues across the lab spectrum. With salicylsulfonic acid, you get to skip those battles. Still, certain labs cut corners, thinking all acids form smooth solutions in water, but that thinking backfires as soon as they run into a tricky compound. As more educational materials highlight properties like solubility before anyone steps into the lab, mistakes taper off. Back in the day, we learned as much from mishaps as from textbooks, but these lessons stick—never treat solubility as an afterthought.
Clear water solubility also means you skip harsh solvents and avoid extra waste. That makes a chemical safer for beginners and seasoned techs alike. Over the years, I’ve seen departments cut down on both waste disposal costs and lab accidents by preferring well-behaved, water-soluble acids. If there’s a choice, it makes sense to pick materials that blend in safely and predictably.
Salicylsulfonic acid’s ready dissolving in water saves time, supports accuracy, and limits safety headaches. These aren’t tiny details; in real practice, they build the foundation for trust in everything from hospital labs to teaching classrooms.
| Names | |
| Preferred IUPAC name | 2-Hydroxybenzenesulfonic acid |
| Other names |
SSA Sulphosalicylic acid Sulphosalicylic acid hydrate Salicylsulphonic acid |
| Pronunciation | /ˌsæl.ɪˌsɪl.sʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | [97-05-2] |
| Beilstein Reference | 1739614 |
| ChEBI | CHEBI:31962 |
| ChEMBL | CHEMBL1591989 |
| ChemSpider | 13074 |
| DrugBank | DB01839 |
| ECHA InfoCard | 17b2b0eba9d-49d9-44a4-bb41-6d35afc59904 |
| EC Number | 211-183-1 |
| Gmelin Reference | 6814 |
| KEGG | C01737 |
| MeSH | D011073 |
| PubChem CID | 11450 |
| RTECS number | VV8225000 |
| UNII | ZR6W6RVF2K |
| UN number | UN2585 |
| CompTox Dashboard (EPA) | DTXSID9044382 |
| Properties | |
| Chemical formula | C7H6O6S |
| Molar mass | 332.32 g/mol |
| Appearance | white crystalline powder |
| Odor | Odorless |
| Density | 1.485 g/cm3 |
| Solubility in water | Soluble |
| log P | -0.72 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.2 |
| Basicity (pKb) | 1.58 |
| Magnetic susceptibility (χ) | -74×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.619 |
| Viscosity | Viscous liquid |
| Dipole moment | 5.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 164.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –1206.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1690 kJ/mol |
| Pharmacology | |
| ATC code | S01GX04 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes severe skin burns and eye damage. Causes serious eye irritation. Harmful if inhaled. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| NFPA 704 (fire diamond) | 3-2-0 |
| Flash point | 185 °C |
| Autoignition temperature | 170 °C |
| Lethal dose or concentration | LD50 oral rat 700 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 700 mg/kg |
| NIOSH | WA2625000 |
| REL (Recommended) | 1 mg/m³ |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
Salicylic acid Benzenesulfonic acid Aspirin p-Toluenesulfonic acid Sulfanilic acid |