Research into isoquinoline alkaloids stretches back well over a century, marking out a key field for both synthetic and natural chemists. The backbone of 1,2,3,4-tetrahydroisoquinoline gained popularity after breakthroughs in total alkaloid synthesis, and the specific design of (S)-3-benzyloxycarbonyl derivatives grew as pressure mounted to develop stereospecific pharmaceuticals. The demand for improved chiral building blocks spurred advancements in protection-deprotection strategies, as well as new salt forms for improved solubility profiles. Early reports on this kind of compound encouraged entire generations of synthetic organic chemists to confront the thorny problem of controlling asymmetry, and the work has paid dividends as more precise, cleaner routes have become available, leaving fewer impurities and granting purer, more reliable products. These developments set the foundation for nuanced work in medicinal chemistry as the pharmaceutical world leaned heavily on chiral starting materials.
(S)-3-Benzyloxycarbonyl-1,2,3,4-tetrahydro-isoquinolinium 4-methylbenzenesulfonate, often shortened to a more manageable name among researchers, sits at an interesting intersection of synthetic chemistry and pharmaceutical preparation. This compound serves multiple functions as a protected amine source, offering both chirality and a removable benzyloxycarbonyl (Cbz) protecting group. The significance lies in its role during stepwise chemical transformations, as it facilitates both lab and industrial-scale production of more complex molecules, especially those where stereochemistry makes or breaks biological activity. The 4-methylbenzenesulfonate, or tosylate, counterion improves crystalline qualities, storage stability, and handling, making this salt form popular for those seeking reliable downstream chemistry.
This compound typically appears as a crystalline or powdery solid, off-white to pale yellow, depending on minor batch variations. Its melting point usually hovers near 140-150 °C, reflecting strong ionic interactions and the robust aromatic system present in both the isoquinolinium and tosylate components. Solubility skews towards polar organic solvents such as methanol, ethanol, and acetonitrile, while sparing solubility can be found in water due to the sizable organic portions. Careful drying and controlled humidity protect the salt from caking, maintaining its workable, free-flowing nature for extended lab bench periods. Chemically, the molecule balances nucleophilicity at the saturated nitrogen with the electron-withdrawing tendency of the Cbz group, tempering unwanted side reactions during peptide or alkaloid synthesis workflows.
You can expect well-established technical documentation for this intermediate, as suppliers commonly provide assay data, usually greater than 98% purity by HPLC, and detailed impurity profiles—a comfort to anyone running sensitive scale-ups or using automated synthesis platforms. Enantiomeric excess stands above 98%, supported by chiral chromatography and NMR verification, since even minor racemization risks hampering downstream efficacy or safety. Comprehensive labeling flags hazards, regulatory status, and batch-specific analytical data, not just for compliance but for everyday transparency and reproducibility in academic or commercial labs. The inclusion of spectral data—NMR, IR, mass spectra—can extend traceability and simplify troubleshooting unforeseen anomalies during research experiments.
The preparation of (S)-3-benzyloxycarbonyl-1,2,3,4-tetrahydro-isoquinolinium 4-methylbenzenesulfonate follows a series of planned synthetic moves. Protection of the amino group on the chiral tetrahydroisoquinoline with Cbz often proceeds under mild conditions, where basic or slightly acidic catalysis prevents unwanted rearrangements and helps secure the stereocenter. Following this, salt formation with p-toluenesulfonic acid in a polar, non-aqueous solvent yields a crystalline product that can be isolated by simple filtration and dried under vacuum. Lab teams routinely monitor purity at each stage using thin-layer chromatography and HPLC, keeping an eye on potential epimerization or hydrolysis. In-house experience shows that cooling rates and solvent choice can swing yield and crystal quality significantly, underlining the value of process optimization, particularly as you move from test tubes to production vessels.
The benzyloxycarbonyl group offers selective stability—resistant to many standard bases and nucleophiles, yet readily cleaved by hydrogenolysis, rendering the amine available for coupling or cyclization. This protection strategy makes the compound a mainstay in multi-step syntheses targeting complex alkaloid or peptide frameworks. The isoquinolinium portion can support further elaboration at various positions, with electrophilic aromatic substitution, allylation, or even metal-catalyzed cross-coupling expanding the structure’s utility. The sulfonate counterion, usually thought of as passive byproduct, occasionally enables phase-transfer catalysis or improves handling in biphasic systems. Modifying reaction conditions, whether adjusting stoichiometry or introducing chiral ligands, can further shape the suite of accessible analogs, making this salt a versatile springboard for diverse chemotype exploration.
In the literature and catalogs, this compound might appear under several aliases. For ease of reference, names include (S)-3-benzyloxycarbonyl-1,2,3,4-tetrahydroisoquinoline p-toluenesulfonate, (S)-Cbz-THIQ tosylate, or simply Cbz-THIQ TsOH salt among synthetic chemists. Each variation refers to the same core molecular structure but emphasizes different facets for specific disciplines—purification workflows, regulatory filings, or standardization across supplier networks. These synonyms can help bridge communication gaps among research teams, especially given the dense nomenclature of chiral pharmaceuticals and their intermediates.
Handling this intermediate brings standard chemical hazards: inhalation and skin contact risks primarily, thanks to potential dust production and the aromatic sulfonate. Proper personal protective equipment, well-ventilated fume hoods, and routine training on spill containment mitigate most exposure concerns. Storage calls for tightly sealed, labeled containers kept away from direct sunlight and sources of moisture, preserving both purity and flow characteristics. Disposal practices should follow local rules, especially since aromatic sulfonates and organic amines can burden wastewater systems if improperly released. Labs with responsible safety cultures lose fewer days to incident response and run more reliable experimental schedules. Regular audits, updated Safety Data Sheets, and coordinated chemical hygiene protocols back up safe, repeatable usage in any setting.
The main audience for this compound resides in pharmaceutical synthesis, especially those programs centered on chiral alkaloids, CNS-active agents, and peptide-based drugs. Medicinal chemists leverage its enantioselectivity during lead optimization, while scale-up teams appreciate the practical stability of both the Cbz protection and the tosylate salt. Its use extends into academic projects seeking to simulate natural product biosynthesis or probe the influence of substituent variation on biological activity. Our own group has woven it into several total synthesis campaigns, finding that simple access to the chiral amine streamlines complex molecule assembly without the hassle of resolving racemates. In recent years, new application angles cropped up in asymmetric catalysis, with researchers deploying analogs as ligands or chiral auxiliaries to nudge precious metal catalysts toward higher enantioselectivity.
Development work around this class of compounds continues at a steady pace, enriched by better analytical hardware and a renewed focus on green, sustainable processes. Teams are pushing to automate repetitive steps—protection, salt formation, purification—freeing up time for longer-term innovation. Advances in chemoenzymatic methods now offer cleaner, more scalable syntheses of chiral isoquinoline derivatives, reducing associated waste streams. Formulation research aims to incorporate these intermediates into solid dosage forms without sacrificing activity or stability, a key consideration as regulatory scrutiny deepens. Patent literature reveals ongoing effort to tweak the aromatic core, chasing more potent or bioavailable candidates for CNS or oncology indications. Collaboration between academic labs and process chemists shortens the cycle for transforming small-batch bench protocols into robust, production-ready SOPs, closing the gap between discovery and market.
Toxicity studies have focused on both the core tetrahydroisoquinolinium skeleton and the individual components—benzyloxycarbonyl, p-toluenesulfonate, and their byproducts. Acute exposure trials in animals point towards low systemic toxicity in the amounts routinely handled for research purposes, yet chronic inhalation or dermal studies show some localized irritation risk, especially from tosylate dust. Residual solvents from large-scale runs carry greater health implications than the parent molecule, reinforcing the push for stringent quality control and batch monitoring. Regular review of new publications and adverse event reports can spot emerging risks, guiding labs to higher safety standards while keeping day-to-day chemists informed about real-world hazards, not just theoretical dangers cataloged in regulatory documents.
Interest in chiral nitrogen building blocks keeps expanding, especially as personalized medicine and increasingly selective drug targets demand bespoke intermediates. Green chemistry principles push for milder, recyclable methods to generate and use (S)-3-benzyloxycarbonyl-1,2,3,4-tetrahydro-isoquinolinium 4-methylbenzenesulfonate, cutting back on corrosive reagents and solvent waste without losing sight of cost and purity. Industry and academia see more potential in engineered enzymes, turning even the more difficult step of asymmetric induction into an accessible workflow. As automated synthesis platforms gain traction, this salt’s stable, bench-friendly form stands to benefit, reducing operator intervention and scaling seamlessly from milligram to kilogram quantities. Intellectual property battles around specific analogs may shape market dynamics, but the base chemistry remains indispensable wherever stereochemistry and stability shape drug development’s next wave.
Anyone who spends time in an organic chemistry lab recognizes how naming sometimes tells a whole story, long before you ever see the molecule. The title here, (S)-3-Benzyloxycarbonyl-1,2,3,4-Tetrahydro-isoquinolinium 4-Methylbenzenesulfonate, packs quite a punch. Breaking that down from real experience, this is a compound often meeting you during synthesis of complex drug molecules, especially ones built around the isoquinoline skeleton.
Starting with isoquinoline, you deal with a bicyclic system – two rings tied together, one aromatic, one resembling a six-membered piperidine ring. Reduce that aromatic ring, hydrogenate it, and you arrive at the tetrahydro-isoquinoline. That bit, the “S,” pins down the stereochemistry, which means folks in chiral chemistry labs immediately understand this version interacts differently in living systems compared to its mirror image.
Look at that “Benzyloxycarbonyl” piece, commonly known as a Cbz protecting group. If you’ve stepped through any peptide synthesis or worked with amines, you’ve probably used Cbz to block reactivity of an amine group. In this case, it sticks onto the nitrogen of the tetrahydroisoquinoline, making the molecule more manageable and less prone to unwanted side reactions. The benzyl part shows up as a phenyl ring with a -CH2- bridge, hooked to a carbamate group. You get both hydrophobic and slight polarity in one package.
There’s a second big player in the story – the 4-methylbenzenesulfonate, better known as tosylate. Anyone who’s run alkylations or purification steps in a crowded lab recognizes that “p-toluenesulfonic acid” salt formation is a savvy move, increasing solubility or just making a new intermediate easier to isolate. Attach the positive isoquinolinium nitrogen to a tosylate counterion, and you end up with a stable crystalline salt, easier to handle and purify than a charged organic molecule by itself.
Compounds like this crop up as building blocks in the hunt for new drugs, especially those targeting cellular receptors which prefer certain stereochemical twists. Researchers zero in on the S-enantiomer because its mirror-image arrangement can mean the difference between blocking pain or just sitting inactive in the body. That stereochemistry comes from carefully controlled synthesis, where the wrong catalyst wipes out your yield or lumps you with a useless mixture.
Tough organic syntheses lean on robust protecting groups. A benzyloxycarbonyl group gets tacked on in early steps and peeled off near the end after everything else has been built. This avoids messy side reactions that can sap research budgets or derail scale-up. In the pharmaceutical world, time means money, but reliability means someone goes home safe, having got their medication. Reliability often depends on an obscure protecting group holding up under heat or solvent changes.
There’s no denying the scale of waste and solvent use in modern pharmaceutical chemistry. Classic steps involving Cbz or tosylate groups often require strong acids, chlorinated solvents, and columns packed with silica–none of which score high marks on the green chemistry badge. Some labs are already moving toward more environmentally conscious alternatives, like switching to milder deprotection strategies and finding salts that dissolve in water, not just dichloromethane.
Structure matters far beyond textbook diagrams. In real daily challenges, knowing why such protective groups and salt forms exist leads to fewer failed batches and cleaner chemistry benches. The structure shapes function, safety, and the environmental toll of the molecules that underpin our medicines.
Most people connect sodium bicarbonate with baking. Not everyone knows this white powder has found a place in homes, hospitals, farms, and factories. In my own house, it sits on the kitchen shelf, ready to rescue cookies from flatness. The science comes from its ability to release carbon dioxide once mixed with an acid, bringing life to cakes and muffins. While many see that as its main gig, the story doesn’t stop there.
Emergency rooms rely on sodium bicarbonate as an antacid for certain patients. The compound quickly counteracts stomach acid, easing discomfort after a heavy meal or a night of spicy food. Doctors often reach for it in urgent cases of metabolic acidosis, saving lives by balancing a dangerously low pH in a patient’s blood. Hospital staff also use it to keep IV lines clear by controlling acidity, and that isn’t something people hear about at the dinner table.
Growing up, every spring cleaning meant scrubbing sinks and fridges with a baking soda paste. This method didn’t require fancy products or obscure chemicals. It works because sodium bicarbonate reacts with grime, loosening stubborn stains and soaking up odors. Its natural mineral structure means it deals gently with surfaces, unlike harsher products that can scratch or discolor counters. Manufacturers cost-effectively add it to everything from toothpaste to laundry detergents. Reports from consumer groups confirm its safety profile, which most parents appreciate.
Most urban water treatment facilities blend sodium bicarbonate into their process to neutralize excess chlorine and control pH. This approach keeps tap water safe for homes and industry. I’ve volunteered in local fish tanks and ponds, where even hobbyists sprinkle the powder to stabilize water for fragile aquatic species. In larger operations, flue gas treatment depends on sodium bicarbonate to trap pollutants before they reach the air. Research by the EPA backs up the environmental benefits, showing reduced acid rain and lower emissions.
Farmers around the world have a practical reason for stocking sodium bicarbonate. Dairy cows, for example, handle digestive issues and produce more milk when given small doses. The substance works directly in the animal’s stomach, cutting down on excess acidity. Agricultural extension offices promote it as a way to improve crop yields by adjusting soil pH without toxic runoff. This simple fix supports a better harvest year after year.
Demand for cleaner air and safer food keeps climbing. Research teams experiment with sodium bicarbonate as a way to clean up industrial waste. Smaller companies invest in greener products that swap harsh chemicals for this tried-and-true staple. If regulatory groups encourage broader adoption, both households and industries might see lower costs and fewer environmental headaches. From what I’ve seen in community groups and scientific reports, it’s clear that creativity plus this familiar compound can offer answers where modern industry falls short.
ReferencesEvery time a product changes hands, a chain of responsibility gets passed along with it. Take something as straightforward as a chemical used in cleaning or manufacturing. Sure, it looks harmless enough on the shelf, but skipping a label warning or overlooking humidity can end up risking both safety and shelf life. For anyone who’s spent time working in a storeroom or warehouse, spilled powder and warped packaging tell their own story: small mistakes add up fast.
A lot of folks I’ve worked with underestimate how sensitive some products can be to temperature swings. Years back on a factory floor, I saw a shipment go bad just because a thermostat malfunctioned over a weekend. Chemicals broke down, and inventory now worth thousands turned into disposal fees and lost time. Storing products at manufacturer-recommended temperatures isn’t just a hoop to jump through—it saves money, meets audit requirements, and protects health. According to the CDC, improper storage is a leading cause of contamination incidents in labs and production environments. Keeping an eye on climate control systems and logging readings pays off over and over.
Dust, moisture, or cross-contact don’t just ruin products; they put teams at risk. After years around warehouses, I can spot the difference between a place that takes hygiene seriously and one that cuts corners. Stack cardboard boxes too close together, leave spills overnight, and pretty quickly, pests or mold show up. The FDA lists cross-contamination as a root cause for recalls nearly every year. Using dedicated storage for volatile or reactive products, following proper seal and organics protocols, and training staff—these steps build a solid defense.
Not every product belongs in the same type of container. Some call for high-density polyethylene to stand up to acids or solvents; others need steel drums or amber glass to block light and prevent leaching. I’ve seen too many facilities try to cut costs by re-using containers, only to face product loss or safety issues. Reading a spec sheet isn’t enough—I’ve learned to speak with suppliers, ask about compatibility tests, and push for clear labeling right down to the batch number. Transparency not only keeps customers safe but guards a company’s reputation.
Day-to-day routines are where good intentions break down. Employees who handle products need clear instructions and training they actually remember. Safety Data Sheets (SDS) can’t be stuffed in a desk drawer. Everyone should know how to find documentation in an instant. During OSHA visits or insurance reviews, I’ve seen policies save a company from huge fines. A well-communicated storage process doesn’t slow anyone down; it builds trust and efficiency.
Disposal can be an afterthought, but leftover material or outdated stock brings risks if ignored. I recommend setting up a schedule for routine checks and safe disposal practices based on EPA standards. Clear signage, personal protective equipment, and spill kits should be part of every storeroom—too many places skip these steps until they face an accident. Having a plan, practicing drills, and assigning clear roles in an emergency aren’t just for big factories; small businesses benefit just as much.
Specialty chemicals come with long names and even longer safety data sheets—at least, that’s what you’d hope for. (S)-3-Benzyloxycarbonyl-1,2,3,4-Tetrahydro-Isoquinolinium 4-Methylbenzenesulfonate doesn’t roll off the tongue, but the important question has nothing to do with pronunciation. Do people working with this compound get clear guidance to handle, store, and dispose of it? A quick search for common sources like PubChem or ChemSpider doesn’t deliver a full safety data sheet. Even so, a bit of digging sheds some light.
Isoquinolinium compounds and sulfonate salts, on their own, don’t scream danger at first glance. Many relatives feature across pharmaceutical research and intermediate synthesis. Both functional groups see widespread use, especially in drug discovery. That doesn’t mean this combo poses no hazard. Research into quinolines and related isoquinoline chemistry makes it clear—some analogues play nice; others interfere with cells, irritate the skin, or kick off allergic reactions. The 4-methylbenzenesulfonate, or tosylate, usually serves as a counterion, sometimes adding a bit of irritation or causing environmental persistence.
Working in or around chemical labs, I’ve watched how teams react to new or niche compounds. Clear hazard information makes the difference between a safe day at work and a freak accident. Safety data sheets form the staple. In situations where safety sheets are absent—or limited to only one language or country—people improvise. Most will lean on similar compounds, scan for warning signs like odd smells, skin tingles, or powders that stick to everything. This is less than ideal.
Knowledge gaps in chemical safety rarely help anyone. Undocumented risks creep into shipping, storage, or simple bench work. In 2022, the American Chemical Society reported more than 800 lab accidents nationwide. Too many began because a substance “seemed harmless” and nobody checked twice. I remember a fume hood session in grad school, mixing something with a name even longer than this one. The bottle offered no more guidance than a lot number and a hazard diamond half-peeled away. Colleagues and I wore three layers of gloves for good measure. That’s no substitute for real data.
Researchers and suppliers that create compounds with limited safety history find themselves at a crossroads. Either invest in getting toxicity, flammability, and environmental data tested—or risk leaving users in the dark. Industry standards suggest that even one-off substances benefit from at least minimal screening: does it burn, does it irritate, does it linger in the environment? Systems for registering new chemicals (like REACH in Europe) hinge on this kind of work. Even a short summary in a supplier listing, or a QR code linking to a lab study, beats radio silence.
In the meantime, every organization handling specialty chemicals, especially with complex structures, needs to train staff in risk assessment and PPE basics. Encourage a culture where nobody skips the fume hood, where gloves and goggles stay on, and where spills are swept up and logged instead of shrugged off. It’s not just about box-ticking—confidence in safety means less stress, better focus, and much lower odds of an emergency.
Until every bottle comes with clear answers, chemists—both professional and hobbyist—should treat unknowns with the same respect they’d give any unfamiliar flame or spinning blade. There’s rarely such a thing as being too careful.
Purity tells you much more than just how clean a chemical looks. It points to safety, reliability, and whether your work will actually succeed. I’ve seen labs and factories buy a product expecting one thing, then end up with inconsistent results because the purity didn’t match their needs. A purity level marked "analytical grade" (99% or better), "laboratory grade" (often between 95–98%), or "technical grade" (sometimes lower, around 90–94%) draws the line between research that gets published and a string of costly failures.
The top grades—USP, ACS, or pharmaceutical—set the standard for pharmaceuticals, diagnostics, and high-end electronics manufacturing. In food processing, it’s "food grade" or nothing, because anything less opens the door to impurities that can make people sick. Unwanted traces of heavy metals or reactive elements drive up costs for remediation and spark regulatory headaches.
Packaging shapes everything from transport safety to waste. Bulk users—think agriculture, treatment plants, and large factories—tend to order chemicals by the drum (often 25, 50, or 200 liters/kilograms) or the tote. Smaller labs or workshops, especially in research and teaching, pick up 100g or 250ml bottles, sometimes right down to 10g vials for especially hazardous or expensive compounds.
In my experience, clear labeling and tamper-proof seals are just as important as the purity itself. Without confetti-proof packaging and detailed safety data sheets, accidents seem inevitable. I’ve seen students grab the "wrong bottle" because storage shelves were crowded or labels peeled off after a spill. Manufacturers have caught on: tighter seals, color coding, and barcode tracking cut down on those mistakes and make recalls possible when batches turn out wrong.
Every label counts. Without batch numbers, manufacture dates, and purity clearly marked, a product turns into a guessing game. Transparency builds trust with customers and shows you’re not ducking responsibility. In industries like pharmaceuticals, regulatory agencies actually walk into your plant and check if every container can be traced, right back to the original factory lot.
The global chemical market faces new challenges as supply chains stretch across continents and companies must answer for every link. Poorly controlled packaging or fudge-prone purity creates huge headaches, especially when importing or exporting. Smarter digital tracking, tighter certification standards, and stronger penalties for bad actors are all already shaking up the sector.
A big part of the purity and packaging story lands on the shoulders of end-users. It’s not enough for a supplier to keep their chemical clean and properly sealed; users need training and the discipline to check those numbers before every use. I’ve cleaned up enough poorly labeled containers in old storerooms to know that neglect sets the stage for contamination and injury.
Standardizing packaging sizes and pushing for higher purity isn’t just good lab practice—it cuts down on leftover waste, reduces storage hazards, and holds companies accountable. Even if the latest regulations feel tough to follow, the payoff in lab safety and product quality makes it worth every extra step.
Greater awareness and attention to purity and packaging will keep chemicals reliable, workers safe, and the environment protected. That’s something everyone—from a lone researcher to a sprawling refinery—needs to care about.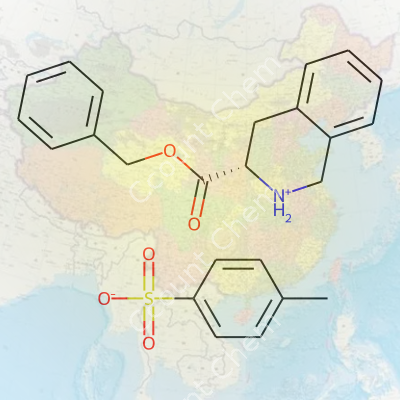
| Names | |
| Preferred IUPAC name | (2S)-3-(Benzyloxycarbonyl)-2,3,4,5-tetrahydro-1H-isoquinolinium 4-methylbenzenesulfonate |
| Other names |
(S)-3-(Benzyloxycarbonyl)-1,2,3,4-tetrahydroisoquinolinium p-toluenesulfonate (S)-3-Benzyloxycarbonyl-1,2,3,4-tetrahydroisoquinolinium tosylate |
| Pronunciation | /ɛs θriː ˈbɛn.zɪl.ˌɒk.siˈkɑː.bə.nɪl waɪ ˈtuː θriː ˈfɔːr ˈtɛ.trəˌhaɪ.drə ˌaɪ.soʊˈkwɪ.nɪ.li.əm fɔːr ˈmɛθ.ɪl.bɛnˌziːn.sʌlˈfəʊ.neɪt/ |
| Identifiers | |
| CAS Number | 162804-26-4 |
| Beilstein Reference | 3738782 |
| ChEBI | CHEBI:131346 |
| ChEMBL | CHEMBL3702047 |
| ChemSpider | 2331626 |
| DrugBank | DB08276 |
| ECHA InfoCard | 43b469c4-80e5-4ee2-ac7a-cf8672c4a902 |
| Gmelin Reference | 85322. |
| KEGG | C16612 |
| MeSH | D017361 |
| PubChem CID | 71305676 |
| RTECS number | This substance does not have an assigned RTECS number. |
| UNII | 1N9R1L1QG3 |
| UN number | Not classified |
| CompTox Dashboard (EPA) | DTXSID7053523 |
| Properties | |
| Chemical formula | C17H18NO2.C7H8O3S |
| Molar mass | 451.53 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.29 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 1.62 |
| Acidity (pKa) | 13.1 |
| Basicity (pKb) | 4.94 |
| Refractive index (nD) | 1.609 |
| Dipole moment | 7.7 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 337.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| Flash point | Flash point: >110°C |
| NIOSH | NA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10g,25g,100g |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1,2,3,4-Tetrahydroisoquinoline 3,4-Dihydroisoquinoline Benzyloxycarbonyl chloride Tosylate Isoquinolinium salts |