Chemistry’s ongoing race to develop safer, more selective, and more effective molecules puts pressure on both academic labs and industry researchers. That’s why the history of (R)-1-(1H-Indazol-4-Yl)-N-(2,2,2-Trifluoroethyl)Propan-2-Amine 10-Camphorsulfonic Acid Salt matters so much. Looking back, the drive to manipulate indazole rings for better bioactivity started decades ago, tied into the early rush on heterocycles in medicinal chemistry. Scientists began eyeing substitutions on the indazole framework as new routes to antitumor, antiviral, or CNS-active candidates. In recent years, adding a trifluoroethyl group boosted metabolic stability and lipophilicity, helping new molecules slip past common metabolic hurdles. The pairing with camphorsulfonic acid draws on classic salt-formation tactics for pharmaceutical intermediates—improving solubility, controlling crystallinity, even enhancing purification steps. Every tweak in this molecular lineage has a story connecting bench innovation and real-world needs, bent on turning chemical possibility into something a little safer or more effective for human health.
Looking at the product, you’ve got a molecule that combines the chiral (R)-enantiomer of a substituted indazole amine with camphorsulfonic acid. The result? A salt form with improved handling and downstream processing. A careful arrangement of trifluoroethyl substitution on the nitrogen fine-tunes activity and binding—trifluoromethyl groups stick around longer in the bloodstream because metabolic enzymes don’t tear through C-F bonds as easily as they do C-H. This can make the difference between a useless compound and a potential blockbuster candidate, especially in the pharmaceutical scramble for next-generation CNS or oncology agents. A well-defined crystalline salt handles better in real-world storage conditions—no surprises from sudden hygroscopicity, no clouds of dust during weighing, better batch-to-batch repeatability. In any research lab, a reliable material means fewer headaches, less troubleshooting, and more predictable scale-ups.
Physical stability isn’t just a buzzword. Good shelf-life, a sharp melting point, and little drift in water or common solvents—these aren’t luxuries, they’re necessities. The trifluoroethyl indazole core offers excellent chemical resilience, thanks to fluorine’s strong electron-withdrawing effects, keeping reactive sites shielded against unwanted side reactions. The camphorsulfonate counterion not only helps with solubility but also tames static and handling problems typical with many free bases or less-robust acid forms. The salt’s particle size and morphology are crucial for weighing, filtering, and even for ensuring reproducibility in multi-step synthesis. Here, you see lessons learned from production-line frustrations: too many batches ruined by clumping, variable density, or pesky polymorphism.
Trust starts with a label—clear stereochemistry, guaranteed purity (usually above 98%), traceability for heavy metals solvents, and consistent crystalline form. A thorough label isn’t window-dressing; people’s safety and patients’ outcomes ride on what’s written there. In regulated sectors, certificates of analysis need to show validated testing for optical rotation, water content, melting point, and residual solvents. Labs depend on the honest communication between supplier and end user. Shortcuts or vague specifications show up soon enough, and not in a good way: failed analytics, inconsistent biological testing, or unpredictable yields in multi-kilogram scale.
The journey begins with the right indazole ring, usually synthesized through classic Fischer indole cyclization or related heterocycle-forming strategies. Chemists then drive a regioselective N-alkylation to install the propan-2-amine tail, taking care to avoid overalkylation or unwanted side products. Next comes stereoselective resolution—usually pulling out the (R)-enantiomer by chiral chromatography or selective crystallization. At this point, the trifluoroethyl group often gets tacked on by nucleophilic substitution or reductive amination, tightening up the molecule’s metabolic profile. Forming the camphorsulfonic acid salt is a straightforward acid-base reaction: dissolve both components in just enough solvent (think methanol or ethanol), warm gently, and let the salt come out in a manageable, filterable solid. Every step along the way reflects countless trial-and-error attempts to balance yield, purity, safety, and environmental impact—especially in a world growing wary of toxic solvents.
You never buy a molecule like this to leave it untouched. Researchers love a scaffold that can absorb substitutions, oxidations, or protection-deprotection swings. The indazole ring still offers handle sites for further modification—halogenation, Suzuki couplings, or direct C-H activation can tune its function for different targets. The amine’s trifluoroethyl group isn’t just dead weight; occasionally, clever chemists use it as a launching pad for further elaboration, swinging from fluorinated analogs to labeled probes, just to see how activity dances across the SAR spectrum. In medicinal chemistry projects, camphorsulfonic acid salt can be swapped out for other counterions when solubility or bioavailability demands change. Modifying, tweaking, or extending the core structure is where real discovery lives, and this compound doesn’t disappoint.
Everyone on the lab floor knows confusion starts with names. Aside from the intimidating IUPAC handle, chemists rely on more approachable synonyms. You’ll see “TFI-propyl-indazole salt,” “R-indazole-CamphSO3H,” and a string of proprietary numbers and supplier codes in catalogs, lab notebooks, and procurement orders. That kind of shorthand, built out of necessity, makes communication easier—right up until crosscheck on ordering gets tangled up by subtle misspellings or swapped numerals. Understanding what each label truly stands for stops dangerous mix-ups and keeps research moving at real-world speed.
No chemical sells without a full set of safety guidelines, and this salt brings its own quirks. The indazole backbone can irritate eyes, nose, and throat with even small accidental exposures, while the trifluoroethyl group means extra caution for skin and mucous membranes. Camphorsulfonic acid, though a favored acid in pharma labs, still carries risk of burns on contact. Proper PPE—nitrile gloves, splash guards, and efficient ventilation—aren’t just bureaucratic hurdles; those rules come from far too many near misses with caustic splashes and unexpected dust clouds. Emergency protocols, thorough SDSs, and spill kits belong in the prep lab as much as anywhere else. Workers who skimp on these steps create more hazards for themselves and their colleagues—and everyone learns that lesson, if not today, then soon enough.
At the end of the day, this molecule matters for what it lets researchers do. In pharmaceutical discovery, it’s a favored scaffold or intermediate for tweaking biological activity: CNS agents, kinase inhibitors, anti-inflammatory hopefuls—the list goes on. When a trifluoroethylated indazole shows up in a pipeline update, interest spikes because it promises resistance to swift metabolic breakdown, letting more active drug reach its target without losing punch. Outside pharma, applications exist in chemical biology—probe development, target validation, even radiolabeling for PET imaging. Each new use emerges from someone willing to adapt a known framework to an unfamiliar challenge, and that cross-pollination often sparks next year’s innovation.
The world of R&D changes course with every failed trial or unexpected bioassay success. (R)-1-(1H-Indazol-4-Yl)-N-(2,2,2-Trifluoroethyl)Propan-2-Amine salts keep showing up at the interface between chemical stubbornness and biological creativity. A team might pivot this scaffold into yet another CNS series after a promising binding affinity at a new receptor pops up on a screening assay, or a lead optimization cycle banks on the trifluoromethyl group’s resilience. You see global investment into smarter, less toxic reagents for preparing and modifying this compound—high-throughput flow chemistry, greener solvents, more predictive computational modeling—because drug development gets more expensive and more competitive with every passing quarter. Successful programs build around reliable intermediates that handle scale-up and regulatory scrutiny without stalling at unforeseen hurdles.
No compound heads down the drug pipeline without a sharp look at toxicity. Early flags about fluorinated amines warn of unwanted persistence in the environment, and metabolite screening must spot anything that looks like a bioaccumulative threat. Preclinical teams dig deep into off-target activity—liver enzymes, cardiac ion channels, or cellular stress responses—building datasets that help predict human safety long before a molecule ever hits a clinical trial. Toxicity research often drives tough go/no-go decisions; promising activity on paper means nothing if rats or dogs suffer adverse reactions after only a few days of dosing. Even researchers far from pharma care about this, since chemoselective transformations often spill over into waste handling, worker exposure, or regulatory audits.
Molecules like this rarely make headlines, but their impact ripples through every phase of drug and probe design. As computational tools keep getting sharper, predicting which indazole variants might slip past resistance mechanisms or show new selectivity patterns, chemists return over and over to tried-and-true structures with just enough room left to innovate. Demand for greener, safer synthetic methods pulls legacy processes toward new reagents, less hazardous solvents, and more recyclable catalysts, minimizing the environmental and regulatory headaches that once seemed unavoidable. The drive for oral bioavailability, brain penetration, and tougher metabolic stability hasn’t gone away—it just gets harder as traditional tricks get overused. (R)-1-(1H-Indazol-4-Yl)-N-(2,2,2-Trifluoroethyl)Propan-2-Amine 10-Camphorsulfonic Acid Salt stands out as a durable bridge between bench chemistry and tested, trusted intermediates, always adapting to new challenges as they emerge.
It’s tough to miss the amount of research circling around selective serotonin receptor modulators. Most folks outside of medicinal chemistry circle might see the name "(R)-1-(1H-Indazol-4-Yl)-N-(2,2,2-Trifluoroethyl)Propan-2-Amine 10-Camphorsulfonic Acid Salt" and feel immediately lost. Strip down that name, though, and you notice its relationship to the indazole class, a group of molecules that’s become increasingly relevant in drug discovery, especially in psychiatric and neurological research.
In labs, this compound attracts attention for its selective interaction with central nervous system receptors. The indazole structure houses key pharmacophores used for tuning selectivity and potency toward serotonin receptors that help regulate mood, anxiety, and cognitive function. Trifluoroethyl groups lend metabolic stability, allowing compounds like this to stay active longer during in vivo testing. By forming the camphorsulfonic acid salt, chemists find it easier to handle and dose—a straightforward but important step in preparative work.
Let’s be honest—a fair share of early-stage research chemicals never see a patient. Many turn up in screens, fail a few tests, and fade from memory. But molecules shaped with some of the features found in this one have passed early hurdles, pushing deeper into studies around treating depression, schizophrenia, and cognitive impairment in neurodegenerative disease. Serotonin receptors, specifically the 5-HT series, make tempting targets, and tweaking structures like this often leads to improved behavior in animal models before advancing them to human trials.
In the mid-2010s, researchers published studies showing increased selectivity for certain 5-HT receptors, often reporting fewer side effects than older, messier antidepressants. That matters. Medications that modulate serotonin systems often come with tradeoffs—sexual dysfunction, weight gain, fatigue. Spotlighting molecules with indazole cores and trifluorinated side chains gave medicinal chemists space to explore alternatives.
Building on that, these molecules support not just scientific curiosity, but hope for people who haven’t found relief in existing drugs. There’s a human side to this: years spent battling depression, misdiagnosed anxiety, or the complications of Parkinson’s disease. New selective ligands offer another chance—one built from careful, incremental progress.
Labs obsess over purity and stability. Researchers test for off-target effects and tolerability before moving out of preclinical stages. In this industry, even promising candidates get dumped if safety signals appear. I’ve seen companies walk away from compounds after years of effort due to an unexpected issue in a simple toxicity screen. For a molecule like this one, maintaining chemical integrity and reproducibility is non-negotiable.
Teams ramp up for scale only if results back up original findings. That means solid analytical protocols, access to reference standards, and reliable sourcing for every salt form produced. Recent guidance by regulatory agencies like the FDA and EMA force drug developers to dig deeper in their assessment before anything approaches clinical use.
Working in pharma, I watched teams move through discovery, scale-up, and preclinical study with stubborn optimism. The journey from benchtop powder to human trial doesn’t hinge on one property or shortcut. Drug programs draw from solid peer-reviewed evidence, decades of failed and successful syntheses, and a relentless focus on patient needs. Reading about new analogs like this, I get a sense of how every fresh chemical entity pushes us a little further—toward better medicines, clearer answers, and new hope for people waiting too long for relief.
Anyone who’s ever stepped foot in a lab understands that juggling chemical storage goes beyond memorizing a few labels or glancing at a safety sheet. I remember the first time a bottle of reagent leaked on my shelf. That taught me quickly how easily things can go wrong when shortcuts get taken. Storage and handling go straight to the core of safety, reliability, and good science.
Heat doesn’t just change the weather—it transforms the world inside a chemical bottle. Certain compounds, especially organics and pharmaceuticals, break down quickly if left near equipment that pumps out heat. Cold rooms and temperature-monitored fridges aren’t just lab luxuries. They stop decomposition before it starts. Chemicals that survive fine at room temperature should still skip the windowsill. Fluctuations or sunlight turn even "stable" bottles into risks. I’ve seen instructors toss entire stockrooms after a single weeklong A/C failure. Dollars and research both disappeared overnight.
The driest powder turns to mush thanks to a little water in the air. Desiccant jars and airtight seals keep hygroscopic substances dry, even if humidity spikes after a rainstorm. For light-sensitive compounds, an ordinary clear bottle spells disaster. Amber glass or foil wrapping blocks UV rays and slows down reactions you never intended to start. The color of the bottle tells you as much as the label stuck to it. It’s downright routine to see staggered expiration dates, even across the same shelf, depending on how tightly someone sticks to moisture and light guidelines.
No one wants to deal with a chemical incompatibility during a lab fire. Acids lurking too close to bases, or fuels stashed beside oxidizers, have set off more emergencies than anyone cares to count. Storage cabinets labeled by hazard—corrosive, toxic, flammable—aren’t an overreaction. They’ve kept more labs open than any set of gloves or goggles. I’ve watched colleagues sweated out room redesigns after audit failures because walls or doors separated the wrong sorts of bottles. Arranging your shelf by hazard class saves headaches, fines, and sometimes much worse. Local fire codes aren’t just red tape; they keep everybody breathing easy.
Half the near-misses I’ve witnessed come from orphaned vials and mystery powders. Clear, legible labeling and up-to-date inventory lists prevent confusion and chaos. Digital tracking helps, but nothing replaces the habit of writing down the date, contents, concentration, and batch. Easy access to safety data sheets speeds up decision-making in any crisis. If your coworkers can’t read your writing, you’re raising their risk. I like to treat each bottle as if a stranger will handle it after me—odds are, one day that’s exactly what happens.
Risk can’t ever drop to zero in a busy space, but strong habits cut it sharply. Training isn’t just a box to check on the onboarding list. Monthly reviews and hands-on drills help new and experienced people alike spot gaps before trouble hits. Investing in the right furniture—spill trays, proper cabinets, low-height shelving—protects against gravity, a foe that never sleeps. As research grows and experiments change, so do storage needs. Flexibility paired with regular audits keeps old habits from creeping back in. Every safe lab or plant I’ve seen puts time into teaching, not just telling, how to do things right.
Most folks think about product purity like it’s a lab benchmark, but out in the field, those numbers carry real weight. Businesses banking on these chemicals—whether in pharmaceuticals, batteries, or construction—stake their reputation and safety record on what’s inside that bag or drum. A slip in purity? That could mean a trial fails, a machine breaks down, or a batch gets wasted. Every decimal point matters.
Purity isn’t just a score on a graph. It tells you about the presence of unwanted guests—impurities that sneak in during production, transport, or storage. Even a tiny bit of an extra metal, trace solvent, or leftover catalyst shapes how the final device, pill, or coating actually works in the real world. Contaminants often block or alter how a chemical reacts, slows things down, or worst of all, causes unpredictable reactions.
People often talk about ‘chemical characterization’ like it’s something only for researchers or patent lawyers. In fact, it matters on the shop floor, in QA labs, everywhere. Defining what’s present (elements, molecules, forms, and structure) sets the groundwork for true quality. So, how do professionals check this? Classical techniques like NMR, FTIR, and X-ray diffraction dig deep into what a material’s made from. Pair that with mass spectrometry, and you start pinpointing trace materials that could cause issues much later. Chromatography can reveal stubborn organic leftover solvents, which matter in applications like food or electronics.
Let’s remember, chemical purity drops for many reasons. Sloppy sampling, rushed production, or containers that leach materials. Even humidity in a warehouse does its part. For folks on the ground like me, unplanned downtime or a batch’s off-spec result is often traced back to a raw material dodging strict quality control. A manufacturer promising 99.99% purity better deliver—because that .01% can sometimes shut down an entire operation.
Some folks brush off extra characterization as a box to check. In my experience, better data upfront leads to fewer recalls, less downtime, and bigger savings. Think about the progress in water testing after the lead scandals, or the crackdown on fake drugs that skirted through inadequate screening. Real chemical identity checks save lives and dollars.
Stricter rules help, but transparency matters just as much. Suppliers sending over thorough batch reports, including spectra overlays and impurity breakdowns, earn customer trust. More labs are taking open approaches to data, matching notes with clients and encouraging spot tests on raw deliveries.
Real change means not trusting tradition alone but pushing for smarter, regular checks. Automated in-line analysis—scanning for drifting purity in real time—keeps errors from snowballing. Portable spectrometers and smart sensors lower the risk of a bad batch slipping through. Folks should keep asking for detailed characterization before the contract is even signed. In the end, it isn’t just about chasing numbers—it’s about keeping people safe, reducing waste, and making sure the product does what it promises every single time.
Every lab veteran recognizes the unique thrill of combining chemicals, picturing the reaction, and hoping the research pays off. But that moment doesn’t come without its backdrop: safety, toxicity, and handling precautions. Years ago, I watched a well-trained colleague splash a small sample on his skin, thinking it was just another compound he’d dealt with hundreds of times. He shrugged it off. The next day, blisters appeared, and he spent hours at the doctor’s office. That experience drummed home the importance of caring about the hazards behind each substance, no matter how familiar they seem.
Too many stories start with someone ignoring the warning section of the datasheet. Some chemicals, like concentrated acids or organic solvents, bring obvious risks: burns, respiratory problems, or even organ damage. Others lurk quietly on the skin, building up tiny injuries. Take sodium cyanide—trace amounts can be lethal—while formaldehyde fumes, barely noticeable, increase cancer risk over time. The Material Safety Data Sheet, usually called the MSDS, breaks down potential harm. Occupational researchers stress that over 2 million work-related injuries each year in the U.S. come from chemical exposure or improper handling. These are not statistics to brush aside.
Whenever I walk into a new workspace, I scan for emergency showers, eyewash stations, and fire extinguishers. Even if management updates policies each year, real protection comes from habits. Gloves and goggles feel like a hassle, especially on hot days, but no shortcut makes up for a lost eye or a chemical burn. Fume hoods run quietly in the background, protecting everyone from vapors, so checking their airflow before starting a task can mean the difference between casual curiosity and a panicked ER trip. I’ve seen a careless spill on bare arms change a promising research day into weeks of medical leave.
Respect grows stronger with knowledge. Simple rules help: never use your nose or bare hands to judge a substance, never eat in the lab, always label containers clearly, and never assume a chemical is “safe enough” because you’ve used it before. Reliable suppliers and up-to-date datasheets build a foundation for safety. The EPA, OSHA, and CDC publish reviews for thousands of chemicals, listing everything from LD50 values to long-term health effects. Following their updates can feel tedious, yet they save lives and careers.
Accidents rarely happen out of the blue. They follow chains of small oversights. Training should go past the sign-in sheet—hands-on practice, routine drills, and real conversations about what actually goes wrong. Regular audits, not just paperwork, keep everyone honest and alert. Teaching new team members how to identify exposure symptoms, proper disposal steps, and decontamination routines keeps a lab healthy for years. Everyone hears the stories about “old-timers with scars to prove it,” but no one should need a scar to learn.
Safety starts with understanding—every day, every shift, every compound. Sharing stories, updating practices, and never letting convenience override caution keep a lab both productive and human. The simple act of putting on gloves, reading the newest safety sheet, or walking a rookie through a spill drill forms the real backbone of safe chemistry. It’s not flashy work, but it’s the work that matters most.
People like to understand what they’re buying. As someone who has managed sourcing for research labs and handled bulk orders in the chemical space, I’ve learned this the hard way. A supplier listing a product with claims but no documentation? That’s an immediate red flag. Documentation isn’t just a bunch of paperwork. Certificates of Analysis (COA), Material Safety Data Sheets (MSDS), and NMR spectra paint a clear picture and build trust between suppliers and buyers.
COA goes beyond a label on a bottle. It lays out the contents, the quality, and confirmation that a batch meets specifications. I’ve seen teams reject entire shipments because a COA didn’t match up with their requirements. For pharmaceutical manufacturers, purity details on a COA aren’t optional—they can mean the difference between compliance and a recall. Even in R&D settings, knowing the content down to the fraction of a percent can change whether a compound goes into an experiment or gets sent back.
Safety in a lab or factory is not just about gloves and goggles. MSDS tells the story behind each substance—health risks, storage rules, spill protocols. In my own early days unpacking shipments, seeing a chemical arrive without its MSDS was a dealbreaker. Regulations in many countries demand this document, and so do insurance providers. If you don’t know the hazards, you put everyone at risk, from the warehouse crew to the folks at the bench.
NMR spectra aren’t just for academic chemists. Suppliers offering reference spectra can save hours and headaches. Once, our team tackled a tricky synthesis only to realize later that the starting material didn’t match its documentation. A quick check of the NMR spectrum would have flagged the issue before any mistakes cost time and money. Transparent NMR data gives buyers a fighting chance to confirm identity and purity—no guesswork, just results.
Regulators keep raising the bar. The US, Canada, and EU each maintain strict standards for chemical documentation. Suppliers found without MSDS or misrepresenting a COA risk more than a lost sale—they court heavy fines and damage to their reputation. Customers talk; one mistake can set a business back years. From tech startups to established manufacturers, documentation keeps everyone honest.
Some companies turn documentation requests into a lengthy process, stalling shipments or holding up decisions. Forward-thinking suppliers no longer treat these documents as afterthoughts. Offering digital access, QR codes on labels, or dedicated customer portals makes buying easier and reduces frustration. After all, a product might be great, but if someone struggles to check its credentials, most buyers just move to the next listing.
It pays to ask for COA, MSDS, or spectra up front. Not just because rules say so, but because it sets a standard for transparency. If a supplier hesitates, that’s something to remember. In my years around the industry, companies that consistently deliver full documentation build stronger partnerships and tend to earn repeat business. Trust isn’t built in a boardroom—it’s built by showing your cards, every single order.
| Names | |
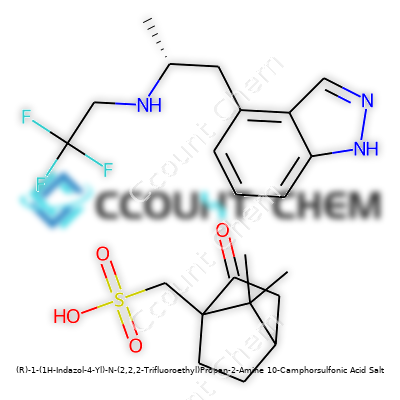
| Preferred IUPAC name | (1R)-1-(1H-indazol-4-yl)-N-(2,2,2-trifluoroethyl)propan-2-amine 10-camphorsulfonic acid salt |
| Other names |
PF-06372865 PF-6372865 Darigabat 10-camphorsulfonic acid salt |
| Pronunciation | /ɑːr wʌn aɪnˈdaezɒl fɔːr ɪl ɛn tuː tuː tuː traɪˌfluəroʊˈɛθɪl proʊˈpæn tuː əˈmiːn dɪˈkem.fər.sʌlˈfɒn.ɪk æsɪd sælt/ |
| Identifiers | |
| CAS Number | 2558905-62-2 |
| 3D model (JSmol) | `3Dmol.js('CC(CNCC(F)(F)F)c1cnn2ccc(C3CC4CCC(C3)S(=O)(=O)O4)cc12')` |
| Beilstein Reference | 11010937 |
| ChEBI | CHEBI:131902 |
| ChEMBL | CHEMBL4533011 |
| ChemSpider | 22228536 |
| DrugBank | DB14539 |
| ECHA InfoCard | 05b498c7-18b0-4eff-851c-e1311349cbee |
| Gmelin Reference | Gmelin Reference: 1465202 |
| KEGG | C20761 |
| MeSH | D014807 |
| PubChem CID | 189078590 |
| RTECS number | This product does not have an assigned RTECS number. |
| UNII | Y1A2F36Q8Y |
| UN number | Not assigned |
| CompTox Dashboard (EPA) | DTXSID40967418 |
| Properties | |
| Chemical formula | C14H16F3N3 • C10H16O4S |
| Molar mass | 422.45 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.38 g/cm³ |
| Solubility in water | Solubility in water: soluble |
| log P | 1.9 |
| Acidity (pKa) | 6.78 |
| Basicity (pKb) | 6.84 |
| Magnetic susceptibility (χ) | -83.6×10⁻⁶ cm³/mol |
| Dipole moment | 4.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 533.6 J/(mol·K) |
| Pharmacology | |
| ATC code | N06AX27 |
| Hazards | |
| Main hazards | H302 + H315 + H319 + H335 |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: – |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/ml |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Indazole derivatives Trifluoroethylamines Camphorsulfonic acid salts N-alkylated indazoles (R)-propan-2-amines Aryl amine salts |