Potassium sulfamate didn’t just pop up overnight. Folks first started seriously exploring it over a century ago during a wave of chemical discoveries that shaped modern lab work and industry. Science students sometimes stumble across its older cousin, sulfamic acid, which saw use in cleaning and weed control, but potassium sulfamate carved out a niche after researchers swapped the acidic hydrogen for potassium. This shift created a compound easier to work with in certain reactions and less corrosive than its acidic predecessor. Its development is tied closely to the needs of the agrochemical sector and analytical laboratories, especially as the push grew for more selective and less environmentally persistent compounds.
Potassium sulfamate comes as a white, crystalline powder that’s highly soluble in water. Simple on the surface, but this property puts it in handy places where quick dissolution is critical. Users notice the salt notably lacks the strong odor that trails behind many potassium compounds. It gets packed and shipped in heavy-duty bags or drum containers, often with minimal fuss, but regulations make sure labeling spells out all hazard and handling info.
Looking at potassium sulfamate, its chemical formula (KSO3NH2) gives a hint at its roots: potassium, sulfur, oxygen, and an amide group. Its molar mass lands around 137 grams per mole. Dissolving this salt in water is a breeze—the process hardly gives off heat, and the resulting solution sits at a neutral to slightly basic pH. It melts at roughly 215°C but doesn’t catch fire easily or release nasty fumes under routine conditions. From firsthand lab experience, it resists most oxidizers but reacts with strong acids, which is pretty typical for sulfamates. It tends to stay stable even if storage conditions fluctuate.
Manufacturers lean into purity here. Specs often require 98% or greater purity, with moisture content capped below 0.5%, and limits on chloride and heavy metals down in the parts-per-million range. Labels stay straightforward: product name, lot number, production date, expiration date, and hazard information under GHS standards. This attention to detail doesn’t just come from regulations—it’s what lets users trust what’s in the bag, especially in agriculture or pharmaceuticals, where quality control matters.
To make potassium sulfamate, producers typically react sulfamic acid with potassium carbonate or potassium hydroxide. This neutralization churns out potassium sulfamate and water—no exotic catalysts or dangerous byproducts. Often, it happens in a big stainless steel reactor, with pH and temperature under steady watch. Once the reaction finishes, the product gets filtered, dried, and milled to the desired crystal size. Waste streams come mostly as rinse water and bit of sludge—still, those streams require treatment to capture any excess potassium or sulfamate left over, especially in regions with strict wastewater rules.
Potassium sulfamate works as more than a bystander in reactions. Unlike potassium chloride or nitrate, it steps in as a mild base and sometimes as a nucleophile in organic syntheses. Some researchers use it to introduce a sulfonamide group to aromatic rings; it does this without the harsh byproducts that come from stronger reagents. Chemists know that under heat and with the right catalyst, potassium sulfamate provides a platform for building bulkier molecules—making it an attractive tool in pharmaceutical development and fine chemicals. Modifications often focus on swapping the amine for bulkier groups to change its reactivity or solubility.
Potassium sulfamate doesn’t hide under many aliases, but you still find it called potassium amidosulfonate or potassium amidosulphamate in British texts. Some older labels shorten it to “KSulfam” or use less common trade names, especially in weed control products. This kind of naming causes headaches in stockrooms since a compound can rest on a shelf under one label while someone searches for it by another. Consistency here matters for safety checks and inventory management.
Most safety guidelines pull from studies on sulfamates in general, since potassium sulfamate itself ranks low in acute toxicity. It doesn’t burn skin or cause much irritation on contact, though inhaling dust over long periods might tickle the throat—making good ventilation and dust masks standard in bigger operations. Spills go straight into waste bins; clean-up routines don’t require hazmat suits, but eye protection, gloves, and careful handling keep workers safe. Storage asks for dry, sealed containers away from acids—the risk ramps up not from explosive hazards but from the stuff caking or reacting with moisture. Plant safety routines pull from OSHA and REACH, so workers follow guides built from hard-earned experience across labs and warehouses.
Potassium sulfamate shines as an herbicide, especially where woody brush or stubborn broadleaf weeds challenge traditional weedkillers. Groundskeepers use it to clear pathways and prep railway embankments because it hits roots deep but breaks down without lingering in soil or water. Some specialty labs tap its ability to modify organic molecules, especially in medicinal synthesis or polymer modification. In a pinch, it slips into niche roles as a buffer or stabilizer for enzyme solutions because it doesn’t disrupt activity like many other potassium salts. Its range has grown with research, especially as folks search for alternatives to more persistent or toxic agrochemicals.
Researchers push to tweak potassium sulfamate’s solubility and reactivity so it fits into new crop protection formulas or advanced chemical syntheses. Part of the story comes from testing blends with surfactants or other additives, aiming for greater weed control at lower doses. Plenty of work dives into creating derivatives with unique selectivity—especially for controlling invasive plant species or boosting plant health without harming beneficial soil microbes. Investment in these R&D programs grows as regulations tighten around older herbicides and demand for greener chemistry jumps both in labs and on the farm.
Most toxicity research shows potassium sulfamate rates as far less harmful than many common agricultural chemicals. Acute oral LD50 data in rodents push it into the “low hazard” category—numbers clock in around several grams per kilogram, well above fast-acting poisons. Chronic exposure studies paint a positive picture: no evidence of buildup or persistent toxicity in soil or water, and no carcinogenic flags waving from the studies reviewed by environmental agencies. Aquatic life shows some sensitivity, especially in low-oxygen settings, so runoff control keeps making its way into application guidelines. Safety hurdles don’t stop at field trials; regulatory agencies keep tabs on new research as part of ongoing risk assessment.
Potassium sulfamate stands ready for a bigger spotlight in the future of sustainable weed control and specialty chemical manufacturing. With restrictions rising on persistent herbicides and regulations clamping down on products that contaminate water supplies, users and manufacturers alike search for solutions that break down clean and leave few traces. Plenty of potential exists for improved application methods—think micro-encapsulation, slow-release tablets, or combination products that block multiple weed types at once. Chemists see opportunities in fine-tuning its reactivity for new pharmaceuticals or specialty polymers. Markets shift fast, and potassium sulfamate finds staying power in versatility—manufacturers and growers willing to experiment with formulation and technique can tap into benefits that go beyond the white powder’s simple looks. Some day, the compound may hold keys to safer food systems, cleaner waterways, and sharper tools on the chemist’s workbench, all wrapped up in an unassuming package that’s earned its place through steady, reliable performance.
One look at the name “Potassium Sulfamate” and it’s easy to think this is another lab-shelf oddity. The truth is, it provides practical value beyond textbooks and industry jargon. Farmers, builders, and even water treatment experts rely on it, though many rarely hear its name outside technical circles.
I remember walking through a railway yard and seeing thick weeds squeezing through the cracks in the old concrete. Soil, tracks, or even garden patios all deal with stagnation from weeds or moss. Potassium sulfamate shows up as a weedkiller, with a knack for targeting deep-rooted problems like Japanese knotweed and brambles. This isn’t just for appearance — in agriculture and public works, tough weeds steal water, space, and nutrients from useful crops or disrupt train safety. Studies point out its role in keeping railway tracks and farm edges clear, improving both yields and safety.
Rotten wooden fence posts tell a familiar story — nature eating away foundations. Potassium sulfamate gets used to treat timber for fungal protection, reducing waste and the cost of constant repairs. Some of the older methods for wood preservation, like creosote, left toxic residues. Regulatory agencies encourage alternatives that carry less environmental baggage. Potassium sulfamate fits that bill — it breaks down over time and doesn’t hang around to cause trouble for fish or pets.
Clear water running from taps relies on more than just pipes; treatment happens before water reaches homes. Potassium sulfamate helps remove minerals and other contaminants through complex chemical reactions. Inside factories, scale buildup inside boilers and pipes gums up the works, wasting energy and money. Adding this compound helps pull out troublemakers, so equipment runs longer before it needs a maintenance shutdown.
Farming needs more than seeds and sunshine. Potassium is a core plant nutrient — it regulates how plants use water, build strong stalks, and survive stress. Some fertilizers swap traditional potassium sources for potassium sulfamate. Though less common than potassium chloride or sulfate, it brings added disease-fighting properties, helping both potatoes and some fruit crops push through fungus-heavy seasons. This dual action — feeding and protecting — appeals to sustainable growers looking to use fewer sprays and invest in lasting soil health.
With all chemicals, safety tops the list. Potassium sulfamate is no exception. I’ve seen crews use gloves and face covers, especially during mixing. Despite its break-down ability, it shouldn’t wash into rivers unchecked or touch bare skin often. Farms and cities can benefit by training workers, enforcing careful storage, and using just enough to get results. Some researchers even explore biodegradable alternatives, though nothing beats potassium sulfamate for certain jobs right now.
Potassium sulfamate isn’t a magic bullet, but it does bridge the gap between tough weeds, decaying wood, contaminated water, and stressed crops. Every community has its stories of overgrown train yards or households struggling to keep fences standing strong through the seasons. Using chemicals wisely — and knowing which to use and where — measures real progress, not just in output, but in safety, sustainability, and keeping life running a little smoother.
Potassium sulfamate comes up in some industrial and laboratory settings. It works as a herbicide, a scale remover, and even helps with certain chemical syntheses. Some folks might never cross paths with it, but anyone working in a lab, a water treatment facility, or on agricultural projects might spot it on a shelf. Safety matters, and handling unfamiliar chemicals always raises fair questions.
Working in a university lab fresh out of school, I learned how even seemingly mild chemicals demand respect. Potassium sulfamate isn’t what most people think of as dangerous, but it can still cause problems. Contact with skin, eyes, or lungs could produce irritation. Inhaling the powder might sting your throat or nose and leave you coughing. Swallowing it can upset your stomach and might send you running for the sink.
Official sources like the CDC and chemical safety databases point out that the dust irritates sensitive tissue, especially without protection. The manufacturers typically recommend gloves, goggles, and a dust mask or respirator. That advice isn’t just lawyer-speak — those basic barriers can keep tiny particles from sneaking past your body’s defenses.
There’s not much public data about potassium sulfamate causing cancer or reproductive harm. Regulatory groups haven’t listed it as a major hazard; it doesn’t rank alongside chemicals everyone’s heard about, like asbestos, benzene, or formaldehyde. But thinking back to my time around research scientists, the lesson hammered home was always to play it safe unless you know a material inside out.
Long-term risks fade quickly when workers follow sensible precautions. Washing hands, keeping workspaces clean, storing solids in sealed containers, and wearing standard lab gear can prevent pretty much every common exposure. A 2022 industrial guide I read mentioned that companies using potassium sulfamate in cleaning and herbicide blends haven’t seen widespread workplace illnesses tied to it.
Most safety problems sneak in during lazy moments—spills, mixing without thinking, storing next to acids, or using old, crusty jars. Potassium sulfamate isn’t flammable, doesn’t explode, and doesn’t release toxic fumes unless it meets strong acids. Pairing it with these chemicals without a good understanding can cause trouble, producing sulfur dioxide gas that no one should breathe in.
Biggest lesson from working safety patrol in grad school: people skip goggles or gloves more than they’d ever admit. Even one irritating splash in the eye changes that habit immediately. Most workplace injuries happen to folks who know better and just get too comfortable. Taking five seconds for basic protective gear and double-checking labels goes a long way, especially in busy spaces.
Clear labels, up-to-date safety sheets on file, and short monthly reminders keep safety fresh in people’s minds. Chemical handling never stays static—a quick team huddle at the start of a shift or before a new batch keeps everyone on the same page. Encouraging people to share near-misses without punishment helps weed out bad habits and tightens protocols. Supervisors who walk the walk with protective gear and good habits set the best example.
Potassium sulfamate asks for respect, not fear. Solid training, easy access to clean water, and keeping incompatible chemicals apart lets people work with confidence. Respecting the basic rules gives peace of mind, knowing nobody leaves a shift with more than they bargained for.
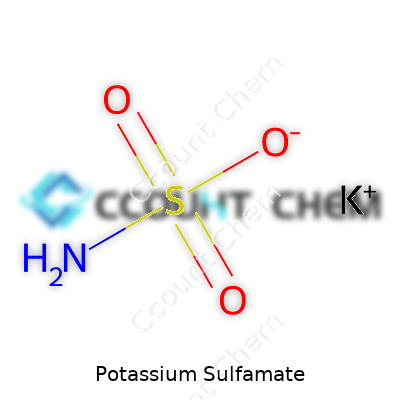
Potassium sulfamate goes by the formula KSO3NH2. This isn’t just a random jumble of letters and numbers. Every element in this formula carries meaning. Potassium takes its spot as K, pulled straight from the periodic table. The sulfamate part hooks up sulfur, oxygen, and an amino group. Put these together, and you get a salt with real-world uses and some chemistry worth talking about.
Reading about chemical formulas may remind some folks of high school labs, but this isn’t just textbook trivia. Potassium sulfamate appears in research, industry, and fields like analytical chemistry. Its specific makeup—due to the balance of potassium, sulfur, oxygen, and the NH2 group—affects its behavior in water, how it reacts, and even its safety profile. Scientists and technicians weigh, measure, and blend exact amounts based on formulas like this one. Having the right formula is as much about safety as it is about science.
Getting a chemical formula wrong causes more than a bad grade on homework. Substituting an atom or mislabeling a group can shift a substance from safe to harmful. In labs, teams trust written instructions. They expect formulas like KSO3NH2 to be accurate because one small slip adds risk. Potassium and sulfur compounds don’t always play nice, so accuracy isn’t negotiable.
Anyone who has worked with chemicals long enough has seen labels swapped or misunderstood. I remember an experiment in school, back before digital scales were everywhere. We almost used potassium sulfate rather than potassium sulfamate. The difference—just a tweak in the formula—would have thrown off the test. Supervisors caught the mistake, but it stuck with me how important the right formula truly is. Real-life situations depend on facts, not guesses.
The chemical world holds no tolerance for lazy shortcuts. Potassium sulfamate’s formula KSO3NH2 is confirmed across trusted sources: reference textbooks, reputable online databases, and scientific literature. It checks out time after time. This isn’t just a scribble on a worksheet; teams rely on these numbers for calculations, safety sheets, and compliance.
Education plays a big part here. Chemistry teachers and peer instructors should drill the value of formula precision early and often. Labs must set up checks before mixing, weighing, or ordering chemicals. Updated labelling standards, clear storage, and a habit of consulting solid references reduce confusion. Companies and universities both can benefit from a culture that rewards double-checking, especially when public and environmental safety enter the mix.
Potassium sulfamate’s formula—KSO3NH2—might look simple. Beneath those letters hides weighty meaning. Every job around chemicals, from academic research to factory production, relies on this kind of baseline accuracy. Cutting corners only brings risk. Precision and careful attention keep people safe while forming the backbone of real progress in science and industry.
Potassium sulfamate doesn’t pop up in dinner table conversations, but anyone who’s ever worked with chemical reagents can tell you it pays to respect the label. Most folks overlook storage, yet chemical safety stories too often start with “someone put it on the wrong shelf” or “the cap was loose after a month.” Schools, hobbyists, and industrial techs run into chemical hazards every day — sometimes because of simple shortcuts, like tucking a container in the wrong place. Speaking from the lab bench, proper chemical storage is all about habits, not fancy policies.
Potassium sulfamate, unlike some of its cousins, doesn’t explode on contact with water or release toxic fumes right out of the jar. Still, it’s far from harmless. Inhaling dust or accidental skin contact can trigger irritation or worse reactions. Take a look at the chemical’s Material Safety Data Sheet and the warnings are clear: keep moisture away, avoid high heat, and separate from incompatible substances like strong acids or oxidizers.
Accidental mixing due to careless storage could endanger everyone nearby. Potassium sulfamate reacts in ways that ramp up risks for fire, toxic gas release, or environmental problems. Too often, folks stack incompatible products together in school cabinets or maintenance closets, assuming that if nothing happens right away, all is well. Science has a way of proving people wrong—sometimes after days, sometimes suddenly.
Rules for storing this compound borrow a page from basic chemistry wisdom. Place containers somewhere dry and cool. Humidity sneaks into loose lids and bags, clumping the product and sometimes starting unwanted reactions. Darkness slows down any decomposition, so skip open shelving near windows. Direct sunlight cooks chemicals fast in sealed containers, and heat speeds up any breakdown.
Use containers with tight seals. Original factory bottles with screw-top lids do a much better job than makeshift jars or worn-out Ziploc bags. I once saw a box sealed with tape that let in so much air condensation formed on the crystals inside. Results weren’t pretty and the mess forced a full clean-up.
Many labs and storerooms organize chemicals by compatibility, which beats alphabetical storage every time. Flammable liquids don’t stand next to oxidizers. Acids stay far away from bases. Potassium sulfamate slots into a section for neutral salts, with glycine and sodium acetate, but on a shelf firmly separated from substances with which it reacts.
People forget that handling storage without training can land them in trouble. New staff or students often rely on common sense, which isn’t always enough. Written policies, regular inspections, and clear labeling prevent most slip-ups. At home or work, anyone handling this chemical ought to know what lives in the next container. Reducing clutter lowers mistakes, and clear signage helps.
Check expiration dates often. Over time, chemical purity drops off even in perfect storage. Don’t hang on to old inventory because tossing chemicals feels wasteful. Local regulations often outline disposal routes — following these keeps both people and the environment safer.
Anyone working with potassium sulfamate needs more than a printout of safety data—they need habits that make dangerous situations less likely. Store it with respect, away from moisture, heat, and the wrong neighbors. Take inventory seriously. Set up shelving by compatibility, not convenience. Teach the next person in line. This level of care keeps risks lower for everyone.
Potassium sulfamate turns up in labs and industrial sites for tasks like herbicide development and specialty cleaning. Anyone handling this compound owes it to themselves and their coworkers to take precautions seriously. A personal story: during my early days in a university chemistry lab, a seasoned supervisor didn’t just hand out safety goggles and gloves; she made us practice donning full gear every time, even if we were just measuring small amounts of similar salts. That habit stuck with me and paid off, since one day, a container cracked mid-weighing. Protective habits gave me time to clean up safely and avoid skin exposure.
Potassium sulfamate can irritate skin and eyes on contact. Keeping direct contact to a minimum means more than just gloves—think lab coats, long sleeves, and proper footwear too. Once, a colleague scooped out a chemical with bare hands by mistake and had to rush into an eye wash station after scratching his nose. Luckily, he suffered only a mild rash, but stories like these remind us how easy it is to overlook small gaps in personal protection. Splash goggles and latex or nitrile gloves create a reliable barrier.
Potassium sulfamate can generate fine dust, especially when poured from large containers. Breathing in this dust carries a risk: coughing, throat irritation, even possible lung discomfort. Nobody wants powder in their lungs—good ventilation makes a big difference. If the room’s air feels stuffy or you see particles floating, a dust mask or a fitted respirator steps up the defense. I’ve seen engineers use local exhaust fans and air-purifying respirators in confined spaces to handle powdered salts, which kept symptoms at bay and helped keep inspections hassle-free.
Storing potassium sulfamate safely means locking it up in a cool, dry spot with a tight seal. Humid air, sunlight, and temperature swings cause clumping and can trigger unwanted reactions with other chemicals. Segregating it from acids and strong oxidizers avoids trouble—combining these by accident means risking toxic gas or unwanted heat. I once spotted a container left open near an acid-wash area, a rookie mistake that led to a stern safety talk and stricter storage practices.
Spills need attention straightaway. Sweeping up dry powder without stirring dust into the air is key; vacuum systems with good filters do most of the work. If potassium sulfamate mixes with a liquid, absorbent pads and plenty of water help wash away residue. Local regulations usually call for treating both solid and liquid waste as hazardous, requiring labelled disposal containers. Years ago, trashing leftover chemicals in the regular bin landed a neighboring lab a fine—and a month of investigation—so now I double-check every waste label before tossing anything.
Chemical safety training pays off the moment something goes sideways. Emergency showers, eyewash stations, and clear labels on chemical containers get overlooked until the moment someone needs them. Frequent drills build muscle memory, so responses in emergencies become instinctive. Sharing safety stories and warnings circles knowledge through the workplace, ensuring everyone knows what to do with potassium sulfamate or any other compound that rolls onto the workbench.
| Names | |
| Preferred IUPAC name | potassium sulfamate |
| Other names |
Potassium amidosulfonate Potassium aminosulfonate Sulfamic acid, potassium salt |
| Pronunciation | /pəˈtæsiəm ˈsʌlfəˌmeɪt/ |
| Identifiers | |
| CAS Number | 10033-21-3 |
| Beilstein Reference | 100817 |
| ChEBI | CHEBI:76210 |
| ChEMBL | CHEMBL572039 |
| ChemSpider | 62948 |
| DrugBank | DB14683 |
| ECHA InfoCard | 14c591d9-3da0-462a-98e7-3dc13c370c7e |
| EC Number | 239-320-4 |
| Gmelin Reference | 14744 |
| KEGG | C18650 |
| MeSH | D011104 |
| PubChem CID | 23668572 |
| RTECS number | WT2450000 |
| UNII | 8H5A36VRT2 |
| UN number | UN2967 |
| Properties | |
| Chemical formula | KSO3NH2 |
| Molar mass | 137.21 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.98 g/cm³ |
| Solubility in water | Soluble |
| log P | -3.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | Acidity (pKa) of Potassium Sulfamate: "5.80 |
| Basicity (pKb) | pKb = 0.80 |
| Magnetic susceptibility (χ) | \-46.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.378 |
| Dipole moment | 3.12 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 132.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −1048.3 kJ/mol |
| Pharmacology | |
| ATC code | V03AB17 |
| Hazards | |
| Main hazards | Harmful if swallowed; causes skin and eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313, P301+P312, P330 |
| Lethal dose or concentration | LD50 (oral, rat): 3200 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2000 mg/kg |
| NIOSH | ST175 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.05 mg/L |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Ammonium sulfamate Sodium sulfamate Calcium sulfamate |