Well before most folks noticed the value of aromatic sulphonates, Potassium 2,5-Dihydroxybenzenesulphonate started quietly shaping lab routines. Its roots trace back to early color chemistry, where synthetic dyes drove research. Chemists experimenting with phenol sulphonation in the 19th and early 20th centuries found that potassium salts helped stabilize products that otherwise gummed up glassware and flouted easy handling. Over time, as textiles and photography demanded faster, more stable reagents, this compound worked its way into the catalogues of European and later global chemical suppliers. By the late 1900s, rising demand in pharmaceuticals and specialty chemicals—think antipyretic agents, dye intermediates, and corrosion inhibitors—brought a spotlight on 2,5-dihydroxybenzenesulphonate as a useful anchor in organic frameworks.
Chemists and engineers favor Potassium 2,5-Dihydroxybenzenesulphonate for its solid handling and straightforward solubility. In my own work, the jump from sodium to potassium salts often led to cleaner filtrations—fewer headaches with caking and better downstream processing. As a white-to-off-white crystalline powder, this salt resists caking unlike tackier alternatives. For process scale-up in intermediate syntheses or pilot plants, users appreciate how easily it dissolves in water, resisting the tendency of some compounds to re-precipitate and complicate separations. Labs and factories alike come back to it because it stores well under dry conditions, not succumbing to the deliquescence that haunts other potassium salts.
Potassium 2,5-Dihydroxybenzenesulphonate carries a molecular weight of about 244 g/mol, a characteristic that comes up in everything from dosing to chromatography planning. Its melting point arrives a bit above 300 °C, with no meaningful decomposition if treated gently. Its white crystals dissolve easily in water—an asset for both synthesis and analytical methods. Chemical structure shows both hydroxyl groups at positions 2 and 5 on the benzene ring, flanking a sulfonate group that links to the potassium ion. This layout grants it mild acidity common to phenol derivatives, along with a touch of surface activity in many water-based systems. The balance of water solubility and ring stability opens doors to both redox reaction work and straightforward salt metathesis.
Commercial shipments often arrive with purity ranging between 98% and 99.5%, based on titration or HPLC area normalization. Labels need to indicate batch number, date of manufacture, content of active ingredient by weight, and origin. Safety data sheets should accompany every drum or bottle, stating eye and respiratory irritancy, along with codes for storage compatibility. Technical documentation helps end-users track contamination issues—trace metal content, water content, and residual organic impurities get flagged before effecting high-value batches. In regulatory terms, labelling ties closely to European and US chemical inventory systems, giving buyers a cross-check on compliance with local standards.
Manufacture usually follows a pattern: benzenesulphonic acid (or its salt) reacts with molten potassium hydroxide in the presence of an oxidant, often under carefully controlled temperature. The process supports formation of the two para-oriented hydroxyl groups on the ring. In my experience, careful pH control and batch-wise addition of KOH prevent runaway exotherms and dark tar formation. Once synthesis wraps up, cooling and simple filtration remove solid impurities, after which solution is evaporated or spray-dried to collect the purified potassium salt. This mode of preparation holds up well to scale, reducing the need for fancy glassware, complicated separation, or rare catalysts.
Potassium 2,5-Dihydroxybenzenesulphonate participates in a surprisingly wide mix of transformations. Nucleophilic substitutions draw on its hydroxyl groups, letting chemists affix alkyl or acyl chains for surfactant or medicinal chemistry use. Oxidation at the ring can yield quinone derivatives—these draw attention in dye chemistry or battery research. Sulphonate remains stable under many conditions, so introducing metals or polymer backbones becomes simpler. In pilot labs, I’ve watched it function as a hydrophilic anchor linking otherwise greasy molecules to emulsifiers or phase-transfer agents. Direct reaction with chlorinating agents lets users swap the hydroxyls for halides, expanding its role as a versatile building block.
Depending on who’s labeling the bottle, this substance might show up as Potassium Hydroquinone Sulfonate, Potassium 2,5-Dihydroxyphenylsulfonate, or Potassium Hydroxyquinol Sulfonate. The CAS number brings clarity in procurement, but trade names sometimes differ—each distributor keen to stand out. Catalogs in the research sector often shorten to K-DHPS or mark it simply as “potassium salt of 2,5-dihydroxybenzene sulphonic acid.” Synthetic chemists trading protocols refer to “potassium hydroquinone sulfonate” interchangeably, especially in English-language journals. Regulatory filings favor the full IUPAC name, but bench scientists lean toward whatever jargon keeps the lab work smooth.
Dust exposure can irritate eyes and respiratory tract, so anyone weighing out the compound should set up local ventilation or wear basic dust masks and goggles. Standard practice includes storing in tightly sealed containers far from oxidizers or acids, since accidental contact can spark off unwanted reactions. Personal anecdote—after one careless spill in graduate school, powders tracked through the lab, and the station needed deep cleaning to avoid residue buildup. In terms of operational standards, regular audits of storage conditions, spill management procedures, and first aid training help protect everyone in the loop. Industry guidelines prioritize documentation; production records track every step, each sample, guarding against batch variation in commercial settings.
This stuff has a broad reach, from dye and pigment intermediates to niche roles in pharmaceutical development. The dye industry prizes it for building blocks in azo and anthraquinone pigments, since the dual hydroxyls and sulfonate both tune colorfastness and water compatibility. Water treatment engineers turn to it when sourcing dispersants and anti-corrosion agents—adding both hydrophilic function and redox buffering. In medicinal chemistry circles, the molecule’s framework stands out for constructing antioxidants or coupling partners in active pharmaceutical ingredients. Academics in electrochemistry and polymer science probe its stability as a testbed for radical scavenging and charge transport, with more patents filed each year as new properties emerge.
R&D teams, both corporate and academic, probe the limits of this sulfonate’s stability and reactivity. My own project a few years back targeted its utility as an anchoring site in self-healing materials, relying on gentle oxidative coupling of the phenol rings. Current literature showcases efforts to improve synthesis yields, leveraging greener solvents and milder oxidants. Work in environmental chemistry weighs the breakdown products and persistence, given increasing concern over micro-pollutants in wastewater. There’s also a push in biomedical research, with teams using its framework to grow complex molecular libraries for screening as drug precursors. This isn’t a dead-end intermediate—it draws sustained attention in the push for sustainability and efficiency.
Preliminary toxicology profiles give it a modestly safe reputation at the lab and industrial scale, but no one takes shortcuts on risk. Acute oral and dermal toxicity sits well below the thresholds of severe hazard, although chronic effects haven’t been as deeply charted outside animal models. Irritation to skin and mucous membranes tends to fall in line with similar potassium salts, leading most handlers to enforce gloves and eye shields during routine work. Environmental toxicology underlines a need for careful disposal—unmanaged releases to water systems might affect aquatic microbial activity, prompting pilot-scale studies on biodegradation and treatment strategies. Compared to many aromatic sulfonates, 2,5-dihydroxy derivatives show promise for manageable toxicity, though continuous monitoring and risk mapping will always be necessary.
Looking ahead, I expect Potassium 2,5-Dihydroxybenzenesulphonate to play a growing role in advanced materials and green chemistry. As industries pivot from volatile organics to water-based processes, this compound’s solubility and predictable reactivity become more attractive. Ongoing research mines its use in battery components, antioxidants, and smart polymers aimed at self-repairing surfaces. Specialty chemical producers pour resources into optimizing production steps: cutting energy use, reclaiming waste, and slashing solvent emissions. Trends in regulatory policy—especially around persistent organic pollutants—push further studies into its environmental fate. As applications keep broadening, manufacturers, researchers, and regulators will keep updating standards and technical guidance, shaping safer, more effective use for the next generation.
Potassium 2,5-dihydroxybenzenesulphonate does not show up in household conversations, yet it touches many products and industries. At its core, this compound stands apart for its unique chemical structure. The two hydroxyl groups and sulfonate group open the door for reactions not possible with simpler molecules. People often notice its impact without ever knowing its name.
Dyers and ink makers took a liking to this compound early on. The molecule’s ability to bond with colorants gives greater shade stability. In printing, companies value how it helps shades stay true, resisting fading under sunlight or routine washing. Textile workers prize fabrics that hold color after repeated runs through the laundry. Consistency matters on the shop floor; clothing that washes out loses customers’ trust. Potassium 2,5-dihydroxybenzenesulphonate helps manufacturers maintain high standards without sky-high costs. Old-school chemistry teachers used to show students firsthand how complex salts provided backbone support for bright and washable dyes.
Photography, before digital cameras took over, depended on careful chemistry. Developers that processed film and print used this molecule to control the speed and contrast of images. Even now, artists who keep darkrooms running look to such chemicals. Certain properties of this potassium salt keep silver particles in balance, reducing fog and preserving detail. What people saw in family photos came thanks in part to careful formulation—simple moments made clear by attention to the science behind light and shadow.
Hospitals and laboratories use many reagents for diagnostics. Potassium 2,5-dihydroxybenzenesulphonate appears in tests for measuring enzymes and sugars in blood. When a doctor reads the results of a liver panel, compounds like this one assured the readings matched the truth. Having confidence in results lets patients and doctors make smart choices. In research, accuracy makes or breaks a study. The same properties that keep cloth bright also keep laboratory values true.
Factories need clean water and controlled reactions. This compound helps remove heavy metals from waste streams, binding with unwanted ions so they can be filtered away. Industries making batteries or electronics find it useful for separating and refining metals. Any misstep in purification can spoil an entire lot, which piles up costs and creates unnecessary waste. By using potassium 2,5-dihydroxybenzenesulphonate, engineers cut down on such risks. Growing up near a river with regular fishing bans taught me the importance of scrubbed factory discharge. A single helpful molecule, put to smart use, can keep water clearer and fish more plentiful.
Every chemical offers benefits and also raises questions about safety and sustainability. Workers handling this material need gloves, goggles, and solid training. Factory owners should invest in regular environmental assessments and transparent reporting. Companies also face demand for greener chemistry. Research into better renewal and recovery gives hope, with more efficient recycling in pilot plants already showing promise. Responsible companies source high-purity raw materials and test their wastewater, building real trust with inspectors and neighbors alike.
Potassium 2,5-dihydroxybenzenesulphonate may not fill headlines, but it gives invisible support to products people rely on every day. Through honest handling and forward-thinking science, its value reaches far beyond the chemistry lab.
Potassium 2,5-dihydroxybenzenesulphonate shows up in industries ranging from dyes and pharmaceuticals to water treatment. Some niche food manufacturing sectors have toyed with related chemicals for their unique properties, but this compound’s name rarely appears on ingredient lists in the grocery aisle. The technical lingo might sound intimidating, but the heart of the question comes down to what science says about putting this chemical in your body.
In my years learning about food science, I always look for two things: official approval and independent research. For everyday supermarket products, the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) publish clear rules on what can go into our food. Potassium 2,5-dihydroxybenzenesulphonate hasn’t received approval as a food additive from either group. I combed through databases—nothing suggests these regulatory bodies consider it safe for direct human consumption.
My search for toxicology reports or clinical research on this compound and edible products didn’t turn up much. What does exist mostly covers its use in chemistry labs, not kitchens. In those settings, researchers treat it like a chemical reagent, not a supplement or preservative. The European Chemicals Agency (ECHA) flags it as needing careful handling—eye irritation, skin warnings, and advice to avoid ingestion. It doesn’t appear in GRAS (Generally Recognized as Safe) lists.
My own cautious nature leans heavily on reliable, proven data. When companies bring a new food additive to market, it takes years of study—animal testing, long-term dietary studies, analysis of breakdown products. Those studies ask whether breakdown products form during digestion, or if people with chronic illnesses could be more sensitive. Every time, someone raises the question: “What if a child ate this every day for years?” For this compound, nobody has filled in those blanks.
Chemicals like potassium 2,5-dihydroxybenzenesulphonate serve important roles outside food. They help clean up industrial spills, or speed up reactions in the lab. I’ve used similar chemicals to dye fabrics or make new molecules for research, but never around food prep. Long words do not automatically mean danger, but a compound made for chemistry sets, not dinner plates, deserves close scrutiny. If it gets into the human diet, by accident or design, there’s no safety net of decades’ worth of human experience.
Safer food comes from clear lines between what’s meant for labs and what’s meant for plates. Companies can protect their customers by sticking to additives with extensive histories and regulatory approval. If anyone wants to introduce a chemical like this into food, a rigorous approval process sets the standard. That means open research, peer review, and public evidence before it enters mass production.
For regular people, the smartest move is to look for recognizable ingredients and keep an eye on regulatory updates. Labels tell part of the story, but transparency and consumer education go further. It’s worth contacting organizations like the FDA or local health departments if questions about any new food ingredient pop up. In my experience, nothing beats knowledge, clear labeling, and a little skepticism about unfamiliar additives.
Potassium 2,5-dihydroxybenzenesulphonate isn’t a headline-grabbing name, but this compound shows up in chemical syntheses, textile processing, and sometimes in research linked to pharmaceuticals. Its stability and behavior keep scientists and technicians on their toes. Chemical safety in the workspace boils down to habits and proven guidelines, not fancy protocol names. I’ve seen labs change for the better after reviewing their own chemical storage, so practices can make or break safe handling of materials like this one.
Dryness sits at the core of safe storage. Humidity creeps up fast, especially in regions with unpredictable weather. Potassium 2,5-dihydroxybenzenesulphonate responds badly to water in the air. The crystals can clump and lose reliability, which means measurements stop lining up with calculated values. Sealing containers tight matters. Using desiccators beats the standard shelf. Simple indicators, like silica gel beads that switch color, come in handy for spotting leaks early. Every chemist I know keeps a stash of those somewhere nearby.
I once caught a batch of sulphonate sitting in a sunlit storeroom. The heat and light can degrade sensitive chemicals long before obvious changes appear. Being careful with temperature and lighting is about more than chemical rules — it means cutting the risk of breakdown or accidental reactions. Room temperature feels broad, but for many lab chemicals, this translates to a cool, shaded shelf, away from direct sunlight and heaters. A locked cabinet under a workbench usually offers better protection than a glass-fronted cupboard.
Cross-contamination does damage quietly. Powdery residues transfer between bottles faster than most people notice. Keeping workspaces clean, storing this compound in its original labeled container, and using fresh scoops aren’t just about avoiding fines; it stops the slow buildup of mystery gunk that can skew experiments or spark surprise reactions. I’ve seen entire projects thrown off by tiny, unnoticed contamination.
Stuff gets dangerous when incompatible chemicals share space. Potassium 2,5-dihydroxybenzenesulphonate reacts if it’s near strong acids or oxidizers. Stashing it far from cleaning products, bleach, or even storage bins marked with hazard stickers matters. OSHA and local guidelines map out these categories, but it’s on the everyday worker to double-check shelves. Training teams to recognize which labels signal trouble side-steps incidents that could halt work for days.
Accidents don’t follow schedules. Spill kits hang on many lab walls for good reason. This compound isn’t especially hazardous by casual contact, but good lab coats, gloves, and eye protection minimize risk. Knowing where the nearest eye-wash and shower stand keeps anxiety low if something goes wrong. Documenting every spill, no matter how small, encourages a culture of transparency that makes storage safer for everyone.
Storage advice only works if people use it. Consistency, habits, and open communication matter more than any self-updating checklist. Potassium 2,5-dihydroxybenzenesulphonate might never make the news, but its storage and handling can teach a team the basics of chemical safety for a thousand other compounds. Regular audits, hands-on training, and clear reminders help keep incidents off the report list — and that’s always worth the extra effort.
People like to trust what they put in their bodies. Most folks check the ingredient labels, search scientific names on their phones, and trust pharmacists to steer them right. Potassium 2,5-dihydroxybenzenesulphonate isn’t exactly a household name, but it shows up in certain medications and sometimes in other chemical products. So, the question of side effects shouldn’t drift through the background unnoticed. Health isn’t meant to play second fiddle to chemistry puzzles.
Science is supposed to shine a light on risks long before anyone tosses a new compound into the mix. The truth here: the scientific record for potassium 2,5-dihydroxybenzenesulphonate feels sparse. Peer-reviewed journal articles and government databases provide only a thin thread about its effects on humans. Most folks working in pharmacology or chemistry rely on public documents, summaries from the European Chemical Agency, and material safety data sheets to flag side effects or hazards.
I’ve double-checked available resources, and it’s clear that potassium 2,5-dihydroxybenzenesulphonate doesn’t come up much in mainstream medical warnings. In large databases like PubMed, no big clinical studies spell out specific adverse reactions in day-to-day use. This either means side effects are rare or reporting simply has gaps. That’s an issue worth worrying about, especially since a lack of info can sometimes mean actual problems sneak past the radar.
Chemical safety relies on patterns. For other sulphonates and chemicals with similar structures, mild irritation can show up if a person inhales or gets them on the skin. Eye irritation makes sense too, given how sensitive the eyes react to almost any chemical. Doctors and laboratory workers usually recommend gloves and goggles around these substances—old habits die hard, but they’re rooted in real experiences.
Individual allergies always make things less predictable. Someone who reacts to related compounds could see hives, swelling, or rarely, more severe symptoms. People with histories of skin sensitivities or breathing issues might want extra caution. There’s no shame in double-checking with a pharmacist or requesting more data before starting any new medicine, especially if an ingredient looks unfamiliar.
People expect companies and public health agencies to share what they know, good or bad. Sticking health warnings in fine print undercuts trust. Every ingredient, not just the famous ones, deserves attention. In my neighborhood, word of mouth spreads fast—one bad reaction, and that story bounces around for years. Only open, evidence-backed updates from health authorities or manufacturers can tamp down rumors.
The right step forward means encouraging deeper research and regular safety audits. Regulators need to check these lesser-known compounds from time to time, not just assume that quiet means safe. Scientists should dig past the basics and publish their findings where everyone can read them. Health staff on the frontlines can flag any odd symptoms linked to potassium 2,5-dihydroxybenzenesulphonate and send those up the chain, making future warnings more reliable.
For people facing a prescription with ingredients like this one, clear communication wins every time. Consulting with healthcare professionals, seeking out up-to-date material safety data, and supporting calls for more research—these practical steps offer real peace of mind in an uncertain field.
Potassium 2,5-dihydroxybenzenesulphonate doesn’t make headlines, but it sits on many lab shelves and in supply rooms for a reason. It pops up in dye chemistry, research, and sometimes as a pharmaceutical ingredient. Anyone who works with chemicals long enough knows the drill—safety isn’t just about avoiding splashes or accidental spills on skin. Mistakes in handling or disposal hang around long after the workday ends. Years ago, I watched a colleague open a poorly sealed container, only to get a noseful of fine particulate. He was lucky, but it taught me chemical handling deserves attention well beyond the basics.
No matter how routine the day feels, treating chemicals with respect never gets old. Gloves, safety glasses, and lab coats come out as soon as the container does. Some skip the mask thinking this chemical isn’t especially volatile. Breathing in any compound, even if it isn’t listed as “harmful if inhaled,” can set off allergies, throat irritation, or worse in sensitive people. A face mask catches airborne bits and provides peace of mind, which, in a busy lab, is priceless.
Storing chemicals matters as much as how you use them. Anyone who’s ever lost a sample to a leaky cap knows frustration, but it’s more than a mess: exposure to moisture or heat degrades purity, creates new risks, or ruins entire batches. Keep potassium 2,5-dihydroxybenzenesulphonate cool and dry, with the container tightly sealed and marked—not just with the name, but with hazard labels. No shortcuts on this front. Shared spaces run into trouble when anything is left unmarked or in a plain jar.
Once the chemical loses its purpose, the real headaches sometimes begin. Throwing it down a drain or into regular trash isn’t just a bad habit; it brings personal and legal risks. Could end up in groundwater or harm aquatic life downstream. Labs face audits and fines for missteps, but most importantly, cleaning up a poorly handled spill costs more than safe disposal ever will.
Following a well-maintained hazardous waste protocol is key. Most institutions hand out safety sheets with rules and contacts. Collect potassium 2,5-dihydroxybenzenesulphonate in a labeled, leak-proof container and call the waste contractor when it’s time. If you work in a small outfit without routine pickups, phone the local hazardous waste service. “Flush with plenty of water” on a material safety data sheet never means ignoring local disposal laws or pouring it down the lab sink.
Plenty of folks laugh off hazard training as box-checking, but every new student or employee needs a hands-on walk-through. No fancy lectures required—just simple rules and reminders to read labels, check supply dates, and never cut corners. After seeing a high school custodian mishandle spilled chemicals, I realized this lesson can’t just live in a powerpoint file or company handbook.
Trust comes from shared responsibility. People who work with chemicals need to speak up about poor practices and suggest fixes. Sometimes, a simple label or extra sign by the sink reminds everyone that proper disposal isn’t up for debate. Supervisors can schedule periodic reminders, keep disposal logs visible, and reward careful attention to safety protocols. The small steps go far—not just in keeping labs up to code, but in protecting coworkers and our surroundings.
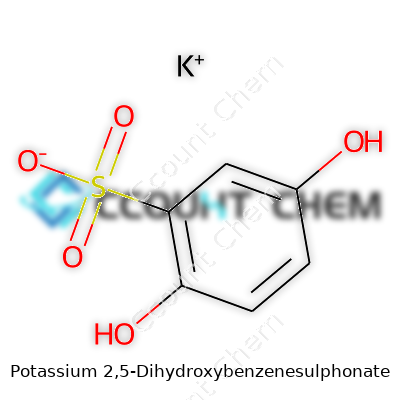

| Names | |
| Preferred IUPAC name | Potassium 2,5-dihydroxybenzenesulfonate |
| Pronunciation | /poʊˈtæsiəm tuː faɪv daɪˈhaɪdrɒksiˌbɛnˌziːnˈsʌl.foʊ.neɪt/ |
| Identifiers | |
| CAS Number | 28305-04-0 |
| Beilstein Reference | 1871755 |
| ChEBI | CHEBI:87617 |
| ChEMBL | CHEMBL2103830 |
| ChemSpider | 21636798 |
| DrugBank | DB13520 |
| ECHA InfoCard | 05d1f080-8579-40df-acf2-91bd47c23ef9 |
| EC Number | 212-611-8 |
| Gmelin Reference | 8885 |
| KEGG | C14356 |
| MeSH | D017371 |
| PubChem CID | 166834 |
| RTECS number | SN6475000 |
| UNII | P8W3J2JJHY |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C6H5KO5S |
| Molar mass | 244.29 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.66 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.95 |
| Basicity (pKb) | 8.05 |
| Magnetic susceptibility (χ) | -65.7e-6 cm³/mol |
| Dipole moment | 2.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 230.0 J·K⁻¹·mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1182.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1615 kJ mol⁻¹ |
| Pharmacology | |
| ATC code | C03EA01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | Precautionary statements: P261, P280, P301+P312, P305+P351+P338, P337+P313 |
| Lethal dose or concentration | LD50 oral rat 4000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral >2000 mg/kg |
| PEL (Permissible) | Not established |