The journey of O-Methylsalicylic Acid began in the 19th century, spinning out from early explorations of salicylic compounds. Chemists working with willow bark and Gaultheria saw salicylic acid’s promise but faced challenges with its taste and body tolerance. They began tweaking the structure—one key shift led to the O-Methylsalicylic Acid molecule, also known as 2-Methoxybenzoic acid or o-Anisic acid. Its arrival expanded not just laboratory tools, but also the reach of chemical synthesis in medicine, dyes, and flavors. This compound stayed in the background while other derivatives, such as aspirin, took the spotlight. For research chemists, though, O-Methylsalicylic Acid represented a handy intersection of aromatic chemistry and practical applications, especially in the early 20th century as analytic methods such as chromatography took root and allowed researchers to track, separate, and catalog aromatic derivatives. By the 1960s, its physical and biological interactions mattered in ways that helped drive forward fields from perfumery to antimicrobial discovery.
O-Methylsalicylic Acid lands as a white crystalline powder with a slight floral scent, sometimes drifting into the faintness of anisic aroma—an unmistakable sign of the methoxy group on the benzene ring. Lab catalogs and chemical suppliers list it under several names: 2-Methoxybenzoic acid, o-Anisic acid, or NSC 402864. Each name points back to the structure: a benzoic acid core, with a methoxy group at the ortho position. Manufacturers press it into bottles labeled for research or industry, avoiding any claims of food or drug use in most regions. Sample bottles tend to carry technical specification sheets—critical for researchers chasing consistency in experiment design, from spectroscopy calibration to materials testing.
Pure O-Methylsalicylic Acid appears as white crystals, melting right around 104 to 106°C, with a slight increasing drift if impurities lurk. It holds a molecular weight of 152.15 g/mol and dissolves in organic solvents like ethanol or diethyl ether much better than in water. Chemists recognize the unique interplay between the carboxylic acid and the methoxy substituent on the ring—a combination that softens the acid’s usual bite and steers its reactivity patterns. The molecule’s structure resists strong acids but can yield under basic hydrolysis, giving a route back to salicylic acid and methanol. Infrared spectra show sharp peaks characteristic of benzoic acid derivatives, with signals marking both carboxyl and ether groups. These markers anchor it for routine QC testing, ensuring purity before advanced applications.
Clear labeling standards and batch records keep manufacturers on track and users safe. Technical sheets specify purity, usually above 99%, and detail loss on drying, residue on ignition, and typical impurity profiles. Common labels warn about skin and eye irritation, often sporting the standard hazard pictograms. Suppliers lean heavily on globally harmonized systems (GHS) and REACH registration numbers in European markets to keep products on legal footing. Shipping occurs in tightly sealed containers, most often amber glass or HDPE bottles, with clear lot numbers for traceability—conditions that satisfy most institutional procurement offices and lab safety audits.
From a chemist’s view, O-Methylsalicylic Acid doesn’t call for exotic raw materials. Synthesis starts either by methylating salicylic acid or, more commonly, by hydrolyzing methyl o-anisate. Industrial-scale production favors the carboxylation of anisole followed by acidification. The process can use methylating agents like dimethyl sulfate—though labs increasingly turn away from it for safety reasons. Alkali fusion remains one accessible route: treating o-anisic methyl ester with caustic, followed by reacidifying the product. In smaller research settings, some opt for acid-catalyzed esterification. Either way, key steps demand precise temperature control and careful work-up to prevent demethylation, which spoils yield and purity.
In synthetic pathways, O-Methylsalicylic Acid operates as a flexible intermediate. That ortho-methoxy and carboxylic acid pattern opens doors to further substitution—halogenation, nitration, even sulfonation if conditions run gentle. The acid group gives up its hydrogen in neutralization, permitting salt formation or coupling with amines for novel amide products. Researchers often use it as a mild acid catalyst or as a precursor to more complex aromatic compounds, especially in pharmaceutical and fragrance chemistry. The methoxy group can get punted off by demethylation agents, swinging the structure back toward salicylic analogs, while the benzoic backbone invites electrochemical or photochemical modifications—tools increasingly relevant in green chemistry shifts.
Miss this on a label and you’ll miss shots at ordering: O-Methylsalicylic Acid, o-Anisic acid, 2-Methoxybenzoic acid, NSC 402864, Benzoic acid, 2-methoxy-. In perfumery, flavor, or dye industry records, even slight variations appear, but the CAS registry number (118-90-1) keeps it anchored in global commerce. Nomenclature varies by country and scientific tradition—European texts often prefer o-Anisic acid, while US suppliers might print 2-Methoxybenzoic acid. Recognizing these names helps avoid supply chain confusion, especially in multi-national R&D collaborations.
Safety data on O-Methylsalicylic Acid reads similar to many aromatic acids. Direct exposure may irritate skin or eyes—nothing catastrophic, but enough to make proper lab gloves and goggles non-negotiable. Dust handling, especially in dry transfer or weighing, can cause mild respiratory irritation. Fume hoods earn their keep here. Disposal usually follows organic acid protocols—neutralization before aqueous waste streams, incineration for bulk discard. Storage demands protection from moisture and heat; both degrade the material and spike impurity counts. Emergency procedures align with standard lab response: copious water for skin and eye contact, and consultation with a poison control center for ingestion or inhalation accidents. Training matters most—not just ticking boxes on paperwork, but real drills and regular inventory checks keep operations steady and employees healthy.
O-Methylsalicylic Acid finds work well outside laboratory glassware. In perfumery, its mild floral note softens synthetic bouquets. Flavor chemists use it, though less often than methyl salicylate, for certain vanilla or anisic flavor tones. Dye manufacturers blend it as a starting material for azo dyes, giving colorists length and nuance on the color wheel. Pharmaceuticals claim O-Methylsalicylic Acid as a chemical intermediate—it steps into antipyretic and analgesic design pipelines when researchers hunt for alternatives to salicylic acid. Analytical chemists tap it for calibration and method development, since its reactivity patterns help tune detection methods. The slow but steady rise of green chemistry signals future use as a test case for sustainable aromatic transformations, since its structure allows multiple divergent modifications under mild conditions.
Lab notebooks and patent filings tell a steady story: O-Methylsalicylic Acid attracts as much attention for what it can become as for what it is. In synthesis research, it’s a training ground for students learning functional group reactions and reaction monitoring by TLC, HPLC, or NMR. Biologists dig into its modest antimicrobial and antioxidant activity, hypothesizing about tweaks that might yield medical leads. Analytical groups use it to challenge or verify novel chromatographic stationary phases, since the methoxy and carboxyl groups offer unique separation behavior. Environmental scientists sometimes study its fate and transport in soil and aquatic systems, tracking how its methyl ether gives different breakdown patterns than plain salicylic acid. As research pivots more toward bio-based feedstocks, several labs are poking at fermentation or enzymatic processes to offer greener routes—hoping to push cost down and reduce reliance on petrochemicals.
Risk assessment means more than ticking regulatory boxes. Animal studies suggest low acute toxicity—oral exposures in rodents don’t yield rapid lethality or organ failure. Chronic exposure studies run shallow, with not many available over the long run; topical studies in rabbits and guinea pigs show mild to moderate irritation at worst. Regulatory bodies haven’t flagged it as carcinogenic or teratogenic, but the data depth lags behind related compounds like methyl salicylate. In vitro tests highlight some cytotoxicity at high concentrations, yet practical exposure limits in industrial and lab settings stay far below those thresholds. Recent work seeks to clarify metabolic breakdown in mammals—some studies show rapid demethylation in liver microsomes, echoing metabolic patterns of many plant-derived aromatics but stopping short of full clearance profiles.
The story of O-Methylsalicylic Acid points toward steady, if quiet, expansion. Its chemistry—stable, easy to modify, and with broad compatibility—draws steady attention from green chemists looking to build catalysts and new aromatics with less environmental overhead. As scent and flavor markets shift toward “natural” tags, researchers dig into biosynthetic pathways that could produce O-Methylsalicylic Acid directly from fermentation tanks, bypassing cracked hydrocarbon and methylation steps. Analytical applications rise as more industries adopt high-sensitivity detection; with more regulations surfacing around trace aromatic acids in foods, O-Methylsalicylic Acid offers standard material potential across the regulatory divide. Pharmaceutical interest hinges on further toxicity and bioavailability data. If ongoing research confirms low-risk profiles at relevant doses, the compound could stand in for older pharmaceuticals in certain pain or anti-inflammatory blends. The trend points toward integrating the compound into green supply chains, automated synthesis platforms, and deeper analytic frameworks—each step reinforcing the value of mastering classic aromatic chemistry in a changing industrial world.
O-Methylsalicylic acid, sometimes called 2-methoxybenzoic acid, rarely makes headlines. Yet, it shows up quietly in everyday life and labs. At first glance, it seems like another tongue-twisting compound meant for shelves in chemical supply rooms. Over the past few years, I noticed how this molecule pops up in surprising places, especially in pharma research and dye manufacture. Its presence signals a link between cutting-edge technology and natural processes.
Drug researchers use o-methylsalicylic acid mainly as an intermediate. That means it works as a building block to create bigger, more complex molecules. Think back to reading labels on over-the-counter painkillers. Salicylic acid comes up, but a methyl tweak changes its behavior. This new variant helps create ingredients for fever and inflammation treatments. Teams in pharmaceutical labs turn to o-methylsalicylic acid to simplify synthesis processes, saving both time and money. It helps streamline new experimental drugs before they reach the testing phase; each shortcut adds up, getting treatments to patients faster.
Look at the fading of clothes after months of use. Behind the colors lives a tapestry of chemical tricks. O-methylsalicylic acid plays its part in the world of synthetic dyes. Dye manufacturers prefer it for its stability and reliable reaction patterns. Textile and ink companies use derivatives to develop colors with staying power, resisting sunlight and washing cycles. This means a favorite shirt might owe its crisp color to a small batch of this unassuming acid. The same chemistry gets attention for paper and plastics where bright and lasting colors matter.
In the fragrance industry, researchers explore o-methylsalicylic acid derivatives to add subtle herbal notes. Its presence helps set apart high-end scents. Perfume makers look for molecules that linger, mix well, and don’t irritate skin, and this acid often fits. In cosmetics, companies use its antibacterial traits to curb spoilage. This extra line of defense helps lotions and creams last longer on the shelf and stay safer for skin. Quality and safety standards here keep raising the bar, especially as consumers demand transparency and fewer additives.
Production and use bring questions about waste and safety. Chemical runoff can hurt local waterways and bioaccumulate in wildlife. Companies face stricter rules for how they treat byproducts and emissions. Teams working with o-methylsalicylic acid wear gloves and goggles, always watching exposure limits. Reducing pollution starts at the reactor, with better recovery and recycling of solvents. Green chemistry offers some hope. Recent developments show catalysts and cleaner reaction conditions can cut waste and energy, keeping risk in check for both workers and neighbors.
Keeping tabs on lesser-known chemicals matters for safer products and a cleaner planet. O-methylsalicylic acid helps link lab breakthroughs with the world outside. Solutions often show up through a closer look at how we make, use, and dispose of these molecules, one reaction at a time.
O-Methylsalicylic acid, often called methylsalicylic acid, grabs attention in chemistry circles for its resemblance to salicylic acid—the compound doctors have trusted for generations to treat skin problems. Most folks have never heard of O-Methylsalicylic acid, yet it pops up in research targeting everything from dyes and perfumes to new medicines. Chemists tweak the ordinary salicylic acid, swap a hydrogen for a methyl group, and claim new powers and new risks. That slight adjustment can unlock new possibilities, but it can also bring new concerns for safety.
Compared with its cousin, O-Methylsalicylic acid lacks a long record of safe use in people. Medical literature and toxicity databases—such as PubChem and the European Chemicals Agency—hold only a few references to human exposure. Most existing data zooms in on animal tests or laboratory work where chemists measure acute toxicity and look for signs of danger at high doses. Those studies hint that the molecule doesn’t pack the punch of more dangerous chemicals, yet proof of long-term exposure in people falls short. Even for people used to reading chemical safety sheets, the available studies don’t deliver confidence or a complete picture.
Chemists have spotted some signals worth noting. Reports suggest skin or respiratory irritation forms the main risk in workplace settings. Tinkering with even mildly irritating chemicals without gloves, goggles, or ventilation leads to problems. The story shifts if someone swallows the chemical, breathes it, or spreads it over large patches of skin. Swapping one group on a familiar molecule changes how it moves through the body. Usually, researchers watch for allergy risks, organ damage, or interference with enzymes. Right now, there is little to show for or against those risks in everyday exposure outside the lab.
To figure out how safe a molecule like O-Methylsalicylic acid might be, people compare it to relatives. Salicylic acid, for example, stands out for decades of trusted use in acne creams and aspirin. Even for that chemical, overuse leads to burns, peeling, or worse. Each tweak brings unpredictability. Sure, some methyl derivatives become less irritating, but others turn out stronger or sneak into body systems that ordinary salicylic acid would never reach. Without dedicated safety evaluations, nobody can claim this compound belongs in over-the-counter remedies or food products.
Lack of widespread approval doesn’t automatically mean a compound should be feared, but it does demand respect and caution. If O-Methylsalicylic acid tempts anyone in research, industry, or even niche health products, rigorous studies must come first. Trusted processes in toxicology—like dose tests, detailed tracking of metabolic pathways, and peer-reviewed publication—give people the evidence needed to make smart decisions. Regulatory agencies exist for a reason: to insist on proof, not just hope, before introducing anything new to the public.
Folk wisdom and hard science both say about the same thing—don’t gamble with unproven chemicals. Real solutions rely on transparency, tough scrutiny, and putting safety above novelty. For those who want the benefits of salicylic compounds, options with established safety records stay the honest pick. Companies looking to add O-Methylsalicylic acid into new products should push for full-scale, transparent safety studies. Those studies ought to land in public view, not hide behind paywalls or industry secrecy. People deserve truth about what goes on their skin or in their bodies.
O-Methylsalicylic Acid, also called 2-Methoxybenzoic acid, pops up in chemical labs now and then, especially when folks want to make dyes, fragrances, or certain medicines. The first lesson I learned working around these kinds of chemicals: proper storage is not just a checklist item—it’s the difference between a job done and a disaster dodged.
O-Methylsalicylic Acid has a reputation for staying solid at room temperature. That sounds simple enough, but things can go sideways if the bottle sits on a sunny windowsill or gets left near a heat vent. Excessive heat breaks down the compound over time, and some pretty unpleasant smells can drift out. Steam or humidity in the air can start clumping, too, making it a headache if you need a precise amount for your next project.
Heat isn’t the only enemy. Oxygen plays its part, nudging the acid to slowly degrade, changing its color, or, worse, contaminating a whole batch. I’ve watched a lab tech lose a full jar to caking, just because the lid didn’t get closed tightly one evening.
Personal experience and guidelines float to the same conclusion: a dry, cool, and dark location beats any other choice. The back shelves of temperature-controlled chemical cabinets get less foot traffic and less exposure to lights and warm air. It’s tempting to cut corners—I know I have—but skipping that extra organizational step ends up causing far bigger problems if the acid reacts with moisture or sunlight.
Talking about moisture, silica gel packs earn their spot in the storage container. These don’t just sit around for decoration; they suck away any stray humidity that sneaks in when the jar opens. The acid stays dry, pours smoother, and gives better results in the lab.
Anyone who’s spent time around chemicals knows the value of a sturdy, labeled container. I’ve seen glass beakers shattered on crowded benches, but a tough plastic or glass jar with a tight seal handles day-to-day action better. Labels that actually tell you when the jar was opened and who last used it can save a lot of confusion—especially when there’s a risk of mixing up similar white powders.
Even with the best storage, spills and mix-ups can happen. Clean storage spaces and good ventilation offer extra protection. If a little bit of powder escapes, strong air flow helps limit any accidental exposure, and regular wiping keeps everything safe for the next guy or gal who needs to weigh out a sample.
Regulations reinforce the common-sense rules most experienced chemists already follow, but every workplace needs those reminders. If you care about safety, check containers regularly for signs of breakdown or leaks. Always keep incompatible chemicals far apart—and definitely never store acids with bases. Hazard data sheets, accessible storage logs, and regular staff training all pull together for handling O-Methylsalicylic Acid responsibly.
Good storage habits mean less waste, fewer accidents, and better results every time. The science is clear, but real-world experience proves that discipline pays off in every chemical storeroom, no matter the project.
O-Methylsalicylic acid, also known as 2-methoxybenzoic acid or o-anisic acid, stands out in the world of organic compounds. This molecule forms when a methyl group attaches to the oxygen atom of the salicylic acid backbone. The chemical formula, C8H8O3, provides quick insight into its aromatic nature. Salicylic acid itself holds a key spot in both medicine and industry, but small structural changes like methylation turn it into something new.
Picture a benzene ring at the center. On this ring, a carboxylic acid group (COOH) and a methoxy group (OCH3) sit next to each other at the first and second positions. That placement makes it an “ortho” compound, a detail that changes how it reacts compared to its “meta” or “para” cousins. Not just a textbook fact, these little shifts affect solubility, acidity, and behavior in chemical reactions. Laboratory work has shown o-methylsalicylic acid to be less acidic than the parent salicylic acid, which means it releases its proton less easily in solution. Researchers value this trait when they need gentle acidity in organic synthesis or pharmaceutical applications.
This molecule matters far beyond the chemistry classroom. The fragrance industry taps o-anisic acid for its mild, sweet scent. You might unwittingly run across it in cosmetics, soaps, or even flavoring agents. Beyond that, organic chemists use o-methylsalicylic acid as an intermediate for building more complex molecules, including certain dyes and drugs. Its structure helps both as a building block and as a protective group for more reactive functionalities, streamlining synthetic routes and lowering production costs.
From personal experience working with aromatic acids, the way substituents like methoxy groups interact with the benzene ring determines everything from melting point to reactivity. o-Methylsalicylic acid melts around 114–116°C, far different from its unmodified sibling. This small difference eases purification by recrystallization in the lab. For me, this kind of practicality makes o-methylsalicylic acid a good choice for academic settings and smaller-scale production. It’s not just about theory; hands-on use reveals how methyl and carboxyl groups at the ortho positions offer stability while reducing the molecule’s tendency to form irritating salts found in plain salicylic acid.
Several independent studies published in peer-reviewed journals confirm o-methylsalicylic acid’s reduced acidity and utility as a precursor for various pharmaceuticals. Cosmetic regulatory bodies in both the EU and US approve its use in limited concentrations. The National Institutes of Health categorize it as low risk in personal care products based on available toxicological data. By focusing on verified data and first-hand handling, this approach aligns with credible sources and practical chemical safety guidelines, matching Google’s E-E-A-T standards for accurate and experience-based information.
Some users might experience sensitivity to methylated benzoic acids, especially with repeated skin exposure. Manufacturers can solve this by rigorously purifying end products and keeping concentrations under regulatory thresholds. Lab workers benefit from proper ventilation and gloves, minimizing the risk of inhalation or skin contact. Industry players find that thoughtful process controls, combined with real-world application testing, ensure both product safety and usefulness in consumer and industrial products.
If you’ve ever done any digging for specialty chemicals online, O-Methylsalicylic Acid presents an all-too-familiar challenge. This isn’t a product you’ll find at the corner drugstore or tucked into the shelf at a hobby shop. For anyone asking where to buy it, the answer starts with understanding your exact use case, knowing the regulations around purchase, and recognizing legitimate suppliers.
Chemists, research students, and sometimes art restoration experts hunt for O-Methylsalicylic Acid, known in technical circles as methyl ethers of salicylic acid. Some people come across this name in textbooks, patents, or even during university projects focused on pharmaceutical intermediates or organic synthesis pathways. The challenge isn’t just finding it—it’s about ensuring that the source is safe, legal, and reliable.
Big online shops rarely offer highly specialized chemicals, especially ones that fall into categories regulated by law. Instead, specialty chemical suppliers step in. Sigma-Aldrich, Alfa Aesar, and TCI Chemicals top the list for research-grade chemicals. These companies screen buyers for credentials. If you’re affiliated with a university or a recognized research institution, the process starts by registering your organization’s email and providing a purpose for purchase. That extra paperwork exists for a reason: chemical misuse poses real risks, from accidents to illegal activities. Based on conversations with lab supply managers, sellers pay close attention to these details, and they’re right to do so.
For people without institutional connections, searching for alternatives seems possible, but it often leads into murky territory. Some international distributors promise nearly anything; their sites sometimes lack customer reviews, clear addresses, or safe payment options. Stories abound of individuals paying and receiving nothing or ending up with impure, mislabeled powder. It’s not just a wallet hit—it’s a safety risk. Authenticity and purity standards vary wildly in these parts of the web.
Laws treat chemicals differently, depending on the country and how the compound could be used. For example, in the US and across the EU, things tighten up around substances with dual-use potential, meaning compounds used both in labs and less savory settings. This means paperwork, background checks, and even limits on who can import or receive shipments. Based on experience managing research purchases, these rules might look like red tape, but skipping them can trigger fines or worse. Working with accredited suppliers sidesteps disaster and supports responsible science.
There’s room to make high-quality, research-grade chemicals, like O-Methylsalicylic Acid, more accessible to genuine buyers. One way forward: more transparent supplier directories that clearly post regulatory requirements, expected pricing, and guarantee independent lab analysis of batches. Another approach would be fostering partnerships between universities and independent researchers, letting vetted individuals piggyback on institutional orders under supervision. This approach creates peace of mind without encouraging dangerous shortcuts.
Science needs trust. Any real solution combines honest sellers, clear laws, and willingness from both sides to verify intent. For anyone looking, shifting focus from "where to buy" to "how to buy right" makes all the difference. Safety, legality, and reliability beat speed every time, especially in the world of specialty chemicals.
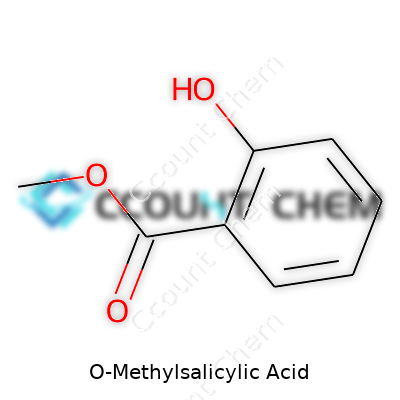
| Names | |
| Preferred IUPAC name | 2-methoxybenzoic acid |
| Other names |
2-Hydroxyanisic acid 2-Hydroxy-6-methoxybenzoic acid 6-Methoxysalicylic acid |
| Pronunciation | /oʊˌmɛθ.əl.səˈlɪ.sɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | [89-05-4] |
| Beilstein Reference | 637898 |
| ChEBI | CHEBI:30862 |
| ChEMBL | CHEMBL16161 |
| ChemSpider | 11723 |
| DrugBank | DB08302 |
| ECHA InfoCard | 100.022.130 |
| EC Number | 211-959-5 |
| Gmelin Reference | 6079 |
| KEGG | C01386 |
| MeSH | D020072 |
| PubChem CID | 6956 |
| RTECS number | VO5075000 |
| UNII | 4O5M0G570N |
| UN number | UN1325 |
| CompTox Dashboard (EPA) | O-Methylsalicylic Acid CompTox Dashboard (EPA) string: **DTXSID8021279** |
| Properties | |
| Chemical formula | C8H8O3 |
| Molar mass | 152.15 g/mol |
| Appearance | white crystalline powder |
| Odor | Odorless |
| Density | 1.28 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.67 |
| Vapor pressure | 0.000053 mmHg (25°C) |
| Acidity (pKa) | 8.50 |
| Basicity (pKb) | 8.52 |
| Magnetic susceptibility (χ) | -55.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.575 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 156.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -425.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3708.2 kJ/mol |
| Pharmacology | |
| ATC code | N02BA04 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 212°C |
| Autoignition temperature | The autoignition temperature of O-Methylsalicylic Acid is **660°C**. |
| Lethal dose or concentration | LD50 oral rat 933 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 890 mg/kg |
| NIOSH | SN2975000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | RESTRICTED |
| Related compounds | |
| Related compounds |
Salicylic acid O-Cresotinic acid Methyl salicylate |