Nickel Bis(Sulphamidate) entered the chemistry landscape when researchers started searching for alternatives to traditional nickel plating solutions. Historical documentation traces early use to labs specializing in metal finishing and electroplating. During the mid-twentieth century, the need to plate objects with improved corrosion resistance prompted scientists to look past simple nickel sulphate or chloride baths. Engineers and chemists collaborated to create new bath compositions, leading to the adoption of nickel sulphamidate-based electrolytes. These developments fueled the metal finishing industry, offering more control over deposit characteristics. Personal encounters show old laboratory notes from the '60s with detailed comparisons between different nickel salts, and sulphamidate options often drew attention for their relative purity and manageable pH profiles.
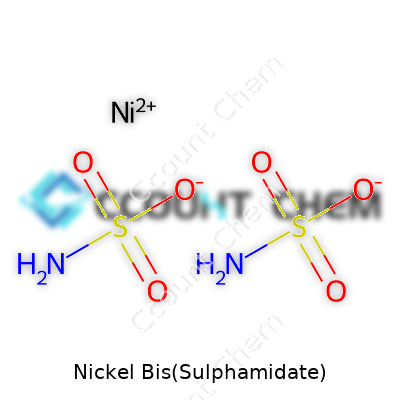
Nickel Bis(Sulphamidate), sometimes listed under names like nickel sulfamate or nickel bis(sulfamidic acid), is a staple in industries that demand smooth, ductile, and low-stress nickel coatings. Chemists and plant operators know this compound as a reliable source of nickel ions for electroplating tanks. Its formula, Ni(SO2NH2)2, lies at the center of discussion when selecting a plating bath. Some manufacturers favor it over other salts thanks to its solubility and its ability to deliver consistent yields in large-batch operations. In my experience, suppliers often highlight this compound for facilities prioritizing high-quality metal finishes on sensitive components ranging from electronics to medical devices.
Nickel Bis(Sulphamidate) forms as a pale greenish salt. It readily dissolves in water, yielding clear, slightly green solutions devoid of the turbidity seen with some other nickel salts. The compound boasts lower internal stress when applied to metal surfaces through electroplating. These attributes matter most to industries where deposit uniformity and resistance to cracking carry enormous value. Chemically, the nickel center is stabilized by two sulfamate ligands, creating a structure that remains robust in a moderate pH environment. Unlike some nickel peroxide or ammonium complexes, this salt doesn’t introduce ammonia odors or unpredictable by-products. In the shop, you’ll encounter tanks full of solution where the metalwork emerges with fewer defects and a surface primed for further processing.
Manufacturers offer Nickel Bis(Sulphamidate) in highly pure forms, usually as crystals or concentrated solutions. Labels display total nickel content, typically measured in grams per liter or as a percentage, and detail information about pH, sulfate impurities, and sometimes even trace heavy metals. Strict quality standards from ISO-certified suppliers lay out batch numbers, expiration dates, and storage recommendations. From experience, shops purchasing in bulk rely on certificates of analysis to match bath make-up guidelines, minimizing risks connected to off-spec product. Packaging uses moisture-resistant containers to ward off caking and accidental hydration before use. On the shop floor, clear labeling cuts down on prep errors which can have ripple effects on finished product quality.
Preparation often starts with high-purity nickel carbonate or hydroxide. A controlled reaction with sulfamic acid under neutral or slightly acidic conditions delivers Nickel Bis(Sulphamidate). Technicians stir the mixture in corrosion-resistant vessels until crystalline product forms, filtering and washing carefully to avoid chloride or sulfate residues. Lab-scale synthesis gives operators more direct control over particle size and purity. For commercial needs, industrial reactors pair tight temperature and pH control to maximize product yield and limit by-products. Personally, I’ve handled scale-ups where diligent monitoring of batch temperature made the difference between a runny solution and the desired crystalline salt. Facilities stress the need for clean water and filtration at every step.
The compound reacts predictably in aqueous environments. It provides a steady supply of nickel ions for electrodeposition with little interference from the sulfamate group. These properties allowed researchers to test modifications using complexing agents or pH buffers. Adjustments with boric acid or leveling agents shape the deposited metal, making it suitable for decorative or engineering purposes. In advanced research, some labs attempted to introduce organic modifiers to nudge grain structure or enhance brightness. Real-life technical reports speak to the challenge of keeping the bath stable while introducing such adjustments, especially during long production runs.
Nickel Bis(Sulphamidate) appears in catalogs under a handful of names. Nickel sulfamate and nickel bis(sulfamidic acid) salt count as most common. You also find listings like bis(sulphamidato)nickel or simply nickel sulphamate hexahydrate, reflecting different levels of hydration or preparation method. These terms sometimes spark confusion for newcomers, but in industry circles, most recognize the product based on the nickel content and intended use in plating baths. Trade names do pop up, tied to specific suppliers offering purity higher than standard or proprietary blending for specialized needs. Past projects sometimes required double-checking formulations when swapping suppliers, since small changes in chemistry affect downstream performance.
Nickel salts demand respect in the workplace. Handling protocols begin with gloves, goggles, and proper ventilation, because inhalation or skin contact risks triggering sensitization and health hazards. Trained staff measure out the salt in isolation from food and drink areas. Many facilities enforce spill kits and run-off controls at the point of use. Transport regulations classify Nickel Bis(Sulphamidate) as a hazardous chemical, so shipping containers carry clear hazard symbols and emergency contact details. My time in production taught the importance of routine air and surface monitoring for nickel, limiting the risk of chronic exposure among staff. Waste treatment processes emphasize neutralization and strict tracking of discharged metals.
This compound stands out in the electroplating world. Automotive, electronics, precision engineering, and medical tooling all benefit from baths based on Nickel Bis(Sulphamidate). Its use delivers layers of nickel that shield parts from corrosion, build up worn surfaces, or create conductive tracks on circuit boards. I’ve seen it underpin the production of springs, valves, and watch components that get exposed to wear and tear every day. Compared with other nickel solutions, nickel sulphamate-based systems let companies fine-tune deposit thickness and internal stress better, leading to improved product lifespan. Downstream, the plated layers take further finishing steps well, including polishing and gold or silver over-plates.
Academic and industrial research continues to draw on Nickel Bis(Sulphamidate) as a tool for innovation. Investigators test new additives and bath conditions to boost brightness, ductility, or environmental compatibility. Universities share results on strategies for reducing hazardous by-products or recovering nickel from spent baths. Collaboration between electrochemists and engineers in facilities around the world advances both the chemistry and applications of this compound. From my collaboration with R&D teams, combining this salt with pulse plating technology pushes the quality of deposit even further, opening doors for microelectronics and MEMS device manufacture.
Nickel’s toxicological profile draws attention from safety agencies and researchers. Nickel Bis(Sulphamidate) shares its parent metal’s risks as a sensitizer, with documented cases of allergic skin reactions and potential respiratory issues. Toxicological studies confirm safe handling limits and propose protective measures, prompting companies to invest in monitoring and exposure controls. Studies also focus on environmental impact, with emphasis on preventing leaching into soil or water around plating facilities. I’ve seen environmental audits drive process changes, from closed-loop recycling of rinse water to more efficient recovery of nickel ions before waste discharge. Ongoing research into less hazardous alternatives highlights the need for continuous vigilance.
Nickel Bis(Sulphamidate) will likely stay central to precision plating, especially in fields where miniaturization and reliability matter. Advances in automation, process monitoring, and green chemistry spur the search for bath additives that reduce health and environmental risks. From industry forecasts and first-hand contact with plating shops, the push for RoHS and REACH compliance accelerates investment in research on cleaner formulations, better waste control, and substitute materials. Labs experiment with hybrid baths that meet changing global regulations, aiming for both workplace safety and product performance. The next generation of eco-friendly electroplating could well spring from the lessons learned refining use of Nickel Bis(Sulphamidate) in daily industry.
Nickel Bis(Sulphamidate) finds its place in rooms that hum with the low buzz of electroplating equipment. Walk into one of those shops, and you might notice someone prepping parts for a run that gives steel a nickel shine. No other compound stands out quite like nickel sulphamate for bright, tough, and even metal coatings. It isn’t the prettiest name, but folks in manufacturing swear by the results.
Dealing with harsh corners or tough threads is a hassle. That’s where this nickel source stands tall. It throws down a layer that sticks tightly and spreads out so well even intricate machines get even coverage. Some industries need that for gears and connectors in electrical setups—think aerospace, automotive, or electronics. Tough, smooth plating helps pieces handle stress, resist corrosion, and avoid sparking trouble later.
You don’t want electronics failing halfway through a job, so the reliability of a nickel layer means less time fighting with repairs and more time getting things done. Nickel sulphamate leaves a cleaner finish compared to the old-school nickel salts often used in past decades. Research from manufacturing journals shows much lower stress on finished parts and fewer cracked coatings, which keeps customers happy down the line.
In my days touring a few local shops, I watched techs plating surgical scissors and precision cutters. Hospitals don’t want mystery metals leaching out and causing trouble for patients. Nickel sulphamate-based solutions help deliver safer, more predictable results that pass tough regulations. The finished tools last longer, so medical staff and patients both win.
Big orders from dental suppliers and orthopedic device companies keep the demand strong. Plating provides an extra barrier against rust—critical where cleaning cycles are aggressive. I learned the hard way how bad a rusty tool can be, so seeing nickel bis(sulphamidate) help reduce those risks feels like progress worth noting.
Nickel isn’t without problems. Long-term exposure to nickel compounds, even in small amounts, can trigger skin issues and respiratory discomfort. Some folks working with plating baths wear thick gloves and masks. Regulations around chemical handling keep getting stricter, but at the end of the day, mistakes still happen. Responsible use—and not taking shortcuts with safety gear—helps prevent accidents.
Wastewater treatment is another hurdle. A shop can’t dump used plating solutions into the drain, so local governments step in and fine violators. Some companies invest in recycling setups that recover nickel compounds from spent solutions. This protects waterways, keeps neighbors happy, and actually saves money over time. Plenty of room exists for more innovation, especially for small and mid-sized operators who want to get greener without breaking the bank.
Nickel Bis(Sulphamidate) isn’t showing signs of fading away. Research pushes for safer alternatives, but today’s demand still relies on its solid, dependable performance. The right use of this chemical lets industries turn out durable, high-quality goods. Keeping safety and environmental impacts top of mind seems like the only way to make sure this tool stays on the right side of progress.
Some chemicals grab attention for their uses in specialized areas, and Nickel Bis(Sulphamidate) happens to play a role in many electroplating shops and laboratory set-ups. Scientists know it for its ability to deliver nickel ions without introducing the headaches that come with other counter-ions. Nickel bis(sulphamidate) owes this reputation to its stability and solubility, traits every chemist wants when laying down nickel coatings for electronics and corrosion protection.
At its core, the chemical formula tells the story: Ni(NSO2NH2)2. To break it down, a single nickel ion joins with two sulphamidate anions. The sulphamidate part, NSO2NH2, steps in with its nitrogen and sulfur to support both the electroplating reaction and long-term solution stability. People who have worked in a plating lab know how fussy some nickel salts can be, leaving unwanted byproducts or even plugging up equipment. This choice, with its simple structure and easy dissolution, makes life easier for both the chemist and the plant operator.
I’ve watched colleagues waste hours troubleshooting baths tainted by secondary reactions. Using nickel bis(sulphamidate) gives them a shortcut—no need to worry about complicated metabolism or breakdowns within common plating systems. Its straightforward formula keeps impurities at bay, helping achieve better, brighter finishes with less maintenance. There’s no major pH swing like there is with nickel sulfate or chloride, so the coating quality stays consistent through long shifts.
Industries trust chemicals that can deliver consistent results. Years ago, I helped set up a nickel plating process for a small electronics manufacturer. They struggled with patchy deposits using an old sulfate system. Swapping in sulphamidate-based chemistry (using Ni(NSO2NH2)2) sorted their problem in days, not months. Their yields rose, reworks dropped, and downtime became rare.
Sure, nickel compounds deserve respect—this one included. Nickel can trigger skin allergies, and waste streams need careful handling. Earning top marks for responsible chemical management means never taking shortcuts. Setting up ventilation, training staff, and using closed systems do more than tick boxes for compliance—they keep people healthy, and every company should be on board with that.
As for the formula, documenting and communicating what’s in use heads off confusion. Too many mishaps trace back to someone not knowing which salt or concentration wound up in a tank. Labeling containers, updating process guides, and sharing material safety data sheets let everyone on the floor stay informed.
Demand for fine, reliable coatings isn’t slowing down. Chemicals like nickel bis(sulphamidate) with formulas like Ni(NSO2NH2)2 put more control back in the hands of operators and lab staff. People get the efficiency, the predictability, and the safety benefits in one package. In the long run, selecting straightforward compounds and teaching their safe use does more for quality and peace of mind than any quick-fix process change.
Nickel compounds often pop up on health watchlists. Nickel bis(sulphamidate) plays a role in industrial plating, electronics, and research labs. Exposure can happen if you are around electroplating tanks or handle nickel salts at work. I once spent a summer at a lab where nickel dust warnings were posted on every wall—those signs weren’t just for show.
Scientific journals and agency reports leave no doubt—many nickel salts cause real health problems. Chronic exposure increases chances of allergic reactions, respiratory trouble, and cancer. The International Agency for Research on Cancer calls nickel compounds carcinogenic to people. Workers exposed for years see higher risks of lung and nasal cancers, and researchers have found allergy rates up to 20% among those with regular contact.
Even without swallowing nickel bis(sulphamidate), simple skin contact is enough to trigger rashes or dermatitis. Itching, redness, even blisters—these aren’t rare stories; they echo through manufacturing floors and labs worldwide. All it takes is a broken glove or poor housekeeping.
Nickel-induced asthma and sinusitis turn up again and again among electroplaters, welders, and machinists. I remember my co-worker’s daily argument with HR about faulty ventilation after his cough refused to go away—eventually, blood tests flagged high nickel levels. The link between exposure and lasting health problems always felt closer than comfortable.
While direct studies specific to nickel bis(sulphamidate) are rare, its close chemical relatives tell a clear story. Skin patch tests with similar salts show allergic reactions in a large chunk of people, especially those already sensitive to cheap jewelry containing nickel. If you’ve ever broken out in a rash from a new watch strap, you know how quick and dramatic these reactions get.
Ongoing health risks from nickel compounds don’t have to become an accepted part of the job. Upgrading ventilation, using closed processing systems, and swapping out old gloves for chemical-resistant pairs make a clear difference. Training teams to spot spills and dust right away pays off. I’ve seen safety initiatives drop nickel exposure by half within a few months.
Testing the air and surfaces for nickel compounds helps catch leaks before anyone gets sick. Respirators designed for fine particulate matter block the kind of dust that sneaks past basic masks. Even small changes matter. I’ve seen labs install simple local exhaust hoods that pulled metal fumes away before they reached anyone’s breathing zone.
Laws on workplace nickel exposure have gotten tighter over the years, but enforcement can lag. Some countries push for stronger chemical substitution policies. Replacing nickel compounds with less-toxic alternatives gives companies a real way to cut risk. Funding more research into specific salts like nickel bis(sulphamidate) helps fill gaps in data and points toward safer practices.
People shouldn’t need to gamble their health for a paycheck. By listening to frontline workers, following science, and investing in smarter protections, we can keep today’s necessary chemicals from becoming tomorrow’s regret.
Plenty of folks assume that chemicals like Nickel Bis(Sulphamidate) act like just another stable solid on a shelf. That trust can backfire. Experience in both research labs and smaller plating operations shows that ignoring the basics leads to trouble—whether it’s lost product, ruined equipment, or putting coworkers at risk. Anyone who has handled nickel salts knows that managing their storage isn’t a throwaway task. People can face skin irritation, lung issues, or in rare cases, lasting toxicity after improper handling or accidental release.
Moisture never helps with shelf life. In humid climates, even a properly sealed drum won’t hold back water forever. Nickel Bis(Sulphamidate) may clump or dissolve into an uneven mess if it sucks in moisture—what started as a smooth-running operation can grind to a halt, sometimes ruining expensive batches of electroplating baths.
A tight, corrosion-resistant container works best. Glass and polyethylene show good results. Store away from acids and oxidizers—old bleach bottles and open acid jugs in the same room crank up corrosion and may even kick off a sketchy reaction, especially in poorly ventilated spaces.
Heat never leaves nickel salts unchanged. After spending years around university labs, I’ve watched storage rooms go from chilly enough to see your breath in the winter to sweltering during a summer blackout. Extreme heat can break down Nickel Bis(Sulphamidate), which might lead to decomposition and inconsistent plating results or even produce toxic fumes.
A cool, dry, and shaded area remains far safer. Common sense says: don’t stick it near radiators, open windows, or in direct sunlight. If temperature swings wildly in your storage area, a simple thermometer and daily log seriously help.
Every young chemist learns totheir peril: unlabeled jars spark confusion and mistakes. Always use a bold, weatherproof label. Include the full name, concentration, and necessary hazard warnings. Think about how often people make quick decisions without checking a database. Clear labeling prevents mix-ups—especially for new staff or folks covering shifts at odd hours.
Keep all nickel compounds separated from organics, flammable materials, and anything you wouldn’t want to mop off the floor during a spill. Having a wall or storage divider, even something simple, cuts down on risk.
A forgotten face shield or ruined pair of gloves leads to real regret. Every storage area containing nickel salts deserves its own PPE locker—nitrile gloves, goggles, dust mask, and emergency eyewash station. Busy workdays tempt shortcuts; everyone gets tired. Placing safety gear within arm’s reach saves skin and eyes in an instant.
Stories from seasoned chemists and plant workers confirm that accidents rarely come from malice or carelessness. Bad habits slip in. A quick refresher on safe storage—perhaps as part of a quarterly team talk—makes a major difference. Fact-based protocols, from ventilation checks to hazard labeling, show respect toward both the product and the people handling it. Keeping Nickel Bis(Sulphamidate) stable for future use is always worth the extra effort.
Nickel Bis(Sulphamidate) pops up often in electroplating shops, chemistry labs, and in some pretty niche manufacturing steps. The stuff can move a project along, but it’s no joke from a safety point of view. People who grew up around industrial chemistry—or even just spent summers working in a plating shop—know that any nickel compound demands respect. You don’t get unlimited free passes if you get sloppy.
This chemical brings two main health threats: skin and lung problems. Anyone who’s ever scrubbed up after a spill or gotten a cloud of powder in their face knows how it grabs your skin or nose and doesn’t let go. Turns out, nickel compounds are linked to contact dermatitis—a rash that itches and burns for days. Inhaling the dust is worse, pushing deep into the lungs with every breath, raising the risk of chronic cough, asthma, and, over time, even cancer. The occupational safety literature calls this out: nobody is immune, no matter their experience.
There’s always someone in every shop tempted to skip gloves or pop their respirator off early. That’s asking for trouble. Good nitrile or neoprene gloves, chemical splash goggles, and snug-fitting lab coats keep this compound off the skin. A well-fitted NIOSH-approved mask or even a full respirator is crucial if you’re scooping powder or working in a dusty spot. A simple T-shirt and wishful thinking don’t block fine particles. If you wind up soaked or exposed, head for the eyewash and safety shower, no hesitation. Acting fast matters more than pride.
A decent fume hood or at least strong local extraction will drag airborne nickel out of the workspace instead of letting it swirl around and collect on every surface. Old shops sometimes skipped this step because it felt like overkill, but air samples from those days show why ventilation changes the game. Regular cleaning, damp wiping, and keeping powder sealed in labeled containers lock in safety. It’s not just about keeping up appearances: nickel residue lingers on workbenches and can wind up on lunchroom tables, inside bookbags, or smeared on doorknobs.
Nickel Bis(Sulphamidate) can’t be rinsed away like soap. Flushing it into the sewer poses toxic hazards for the environment and for people working downstream. Used solutions, absorbent pads, and cleaning rags should all go into hazardous waste drums. Local rules often set strict disposal steps for nickel, and ignoring them lands both health fines and a heavy guilty conscience. In the past, I saw well-meaning workers pour leftover baths down drains, only to face closed fish farms and water warnings months later.
Training isn’t just a formality. Workers who know the symptoms of nickel allergy and early warning signs of breathing trouble catch trouble before it lands them in urgent care. Easy-to-read labels and color-coded containers help new hires and old hands avoid mix-ups. Stories float around of someone grabbing the wrong beaker on a rushed morning—thorough labeling makes those stories far less likely, and safety boards with emergency protocols fill in the rest.
Safety with Nickel Bis(Sulphamidate) doesn’t happen by accident. It grows from habit, training, and stubborn attention to detail. The best shops and labs make safety a team attitude, not a checkbox. Real trust builds when people watch each other’s backs and insist on doing things the careful way, every shift.
| Names | |
| Preferred IUPAC name | Nickel bis(sulfamate) |
| Other names |
Nickel(II) Sulfamate Nickel sulfamate Nickel diaminosulfonate Sulphamic acid, nickel(2+) salt (2:1) Nickel(II) sulfamate tetrahydrate |
| Pronunciation | /ˈnɪk.əl bɪs sʌlˈfæm.ɪ.deɪt/ |
| Identifiers | |
| CAS Number | 12418-60-1 |
| Beilstein Reference | 1461403 |
| ChEBI | CHEBI:86384 |
| ChEMBL | CHEMBL3316372 |
| ChemSpider | 21639197 |
| DrugBank | DB16038 |
| ECHA InfoCard | ECHA InfoCard: 100.031.961 |
| EC Number | 438-670-8 |
| Gmelin Reference | 128142 |
| KEGG | C18625 |
| MeSH | D017252 |
| PubChem CID | 23684736 |
| RTECS number | QR9600000 |
| UNII | A2F83H6N4Z |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | DTXSID20221252 |
| Properties | |
| Chemical formula | Ni(SO2NH2)2 |
| Molar mass | 300.06 g/mol |
| Appearance | Light green crystalline solid |
| Odor | Odorless |
| Density | 3.41 g/cm³ |
| Solubility in water | Soluble |
| log P | -1.44 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 13.2 |
| Basicity (pKb) | 6.30 |
| Magnetic susceptibility (χ) | 4.8 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.62 |
| Dipole moment | 2.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 147.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1016.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –1594 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V07AX10 |
| Hazards | |
| Main hazards | Toxic if swallowed, in contact with skin or if inhaled; may cause an allergic skin reaction |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H301 + H331: Toxic if swallowed or if inhaled. H317: May cause an allergic skin reaction. H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled. H351: Suspected of causing cancer. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): >5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 5,000 mg/kg |
| NIOSH | WA9820000 |
| PEL (Permissible) | 1 mg/m³ |
| REL (Recommended) | 5 – 10 g/L |
| IDLH (Immediate danger) | IDHL: "Nickel Bis(Sulphamidate): 10 mg Ni/m³ |
| Related compounds | |
| Related compounds |
Nickel sulfate Nickel chloride Nickel nitrate Nickel bis(acetylacetonate) Nickel(II) carbonate |