N-Phenylcarbamimidoyl ammonium carbonate hydrate’s history demonstrates the steady march of chemical innovation. Its roots draw from the evolution of guanidine chemistry back in the late 19th century. As attention focused on guanidine derivatives in the world’s great laboratories, chemists sought compounds that could combine guanidine’s famed reactivity with new organic scaffolds. The addition of the phenyl group and formation of the ammonium salt opened up fresh possibilities, and by the late 20th century, new routes to more stable and water-soluble guanidine carbonate compounds shaped specialized research. The hydrate form, bringing in an explicit water of crystallization, increased stability and handling, directly addressing safety and longevity issues encountered with anhydrous salts. This reflects a cycle of discovery where each generation built on practical challenges from its predecessors.
The compound stands out in the family of guanidine derivatives, combining the features of a phenyl ring bonded to guanidine with an ammonium carbonate counterion, stabilized as a hydrate. This amalgamation points to pharmaceutical and chemical utility, particularly where strong organic bases or nucleophiles are critical. In the lab, its solid state allows for convenient weighing and transfer. Bench chemists often value these compounds because handling liquid alternatives gets messy quickly, and hydrate forms make for less dust—a small detail with real significance for those who weigh out hundreds of samples.
N-Phenylcarbamimidoyl ammonium carbonate hydrate appears as a white to slightly off-white crystalline powder, with noticeable bulk density. Its hydration state keeps it stable under ambient humidity, a detail with daily operational consequences in real-world storage. Solubility trends reveal good water dissolvability, but less so in non-polar solvents. The molecule’s combined guanidine core and phenyl substitution gives a melting point higher than unsubstituted salts, adding thermal robustness necessary for applications running above room temperature. Chemically, it stands as a strong base, eager to participate in proton abstraction, but less aggressive than alkali hydroxides, offering more selectivity in organic syntheses.
Manufacturers provide this product with detailed batch analysis, covering purity usually above 98%, moisture content, identification by NMR and IR, and specification of counterion composition. Labels include essential hazard and precaution information aligned with GHS (Globally Harmonized System), warn about skin and eye contact, and note its limited inhalation risks due to hydrate stability. Each vial receives a lot number and storage guidelines. Labs working in regulated industries rely on these traceable records not just for quality assurance, but to comply with audit trails demanded by agencies around the world.
Typical preparations rely on a two-step approach. First, phenylguanidine is treated with excess ammonium carbonate solution, sometimes in mildly basic conditions at cooled temperatures, to favor salt exchange. After forming the desired ammonium salt, slow evaporation or cooling brings out crystalline hydrate. This workup means operators avoid hazardous organic solvents, lean on water-based crystallization, and produce less waste—fitting modern ‘green chemistry’ targets, which matter more than ever across research and industry.
As a highly nucleophilic guanidine derivative, N-phenylcarbamimidoyl ammonium carbonate hydrate participates in a sweep of reactions. It reacts smoothly with acyl halides or activated esters, forming substituted amidine products prized in medicinal chemistry. The phenyl moiety improves selectivity for certain aromatic substitution routes. In heterocycle syntheses, it helps build triazine, pyrimidine, and other nitrogenous scaffolds without cumbersome protecting group strategies. The carbonate counterion can exchange under the right conditions, opening up related salt forms if a process demands different solubility or thermal profiles.
The chemical plays under several aliases—not just ‘N-phenylcarbamimidoyl ammonium carbonate hydrate’, but also titles such as ‘phenylguanidine ammonium carbonate hydrate’ or, in some catalogs, simply ‘phenylguanidinium carbonate’. Each name signals the compound’s core structure: a guanidine with phenyl and ammonium carbonate combination. For procurement, these alternative names mean broader supplier search results and reduced lead time, a practical benefit in clinical trial R&D where waiting weeks means lost opportunity.
Safety expectations for this chemical center around its strong basicity and moderate irritant risk. Reputable suppliers include comprehensive SDSs (safety data sheets) covering potential for skin and eye irritation, advice to use gloves, goggles, and in some cases, a face mask. Inhalation risks lessen substantially thanks to hydration and low volatility. In my experience, the powder remains easy to contain, rarely generating airborne dust under typical conditions. Spill cleanup just means moistening the material, collecting, and sealing for disposal. Training lab workers in good housekeeping and issuing basic PPE reduces nearly all exposure hazards, and regular audits reinforce this culture of safety.
The draw of this compound comes from its value in synthetic organic chemistry. Medicinal chemists prize it for constructing bioactive guanidine or amidine groups common in drugs targeting proteins and nucleic acids. Agrochemical discovery teams adopt it as a building block for plant protection agents. In polymer chemistry, it catalyzes selective reactions where urea or plain guanidine falls short. Academic groups keep it on hand for method development—if you want to test a new reaction, having a reliable base like this speeds up troubleshooting. Its role stays niche but vital, especially in pilot-scale routes aiming for both yields and reproducibility.
R&D teams investigating guanidine derivatives look for compounds with improved stability, water solubility, and reactivity. The hydrate’s engineered properties address many persistent hurdles in handling and storage. Process chemists in pharmaceutical discovery innovate ways to scale up carbonate salt production—chasing better yields, cleaner purity, and easier downstream formulation. Chemoinformatics groups evaluate derivatives for predicted drug-like characteristics, while collaborating with toxicologists to steer away from candidates with unwanted metabolic fates. The cross-talk between labs, suppliers, and regulators sharpens the focus on safe, sustainable synthesis as the sector’s top challenge and opportunity.
Studies into toxicity find a low acute risk, but repeat exposure causes mild skin or eye irritation. Chronic data remain limited, as thorough regulatory studies on established pharmaceutical guanidines often get extended to this compound by analogy. Preliminary work in rodents shows rapid renal excretion, aligning with expectations for polar, water-soluble guanidines. Toxicologists watch for unwanted byproducts during high-temperature reactions, since trace aniline or related phenylamines can present carcinogenicity risks. Laboratories keep careful records, and many have moved towards in-house rapid screening to check for unexpected toxic breakdown under stress. This hands-on vigilance follows from previous chemistry’s hard lessons: better to catch problems in minute quantities than chase remediation years later.
Looking ahead, interest grows in tuning N-phenylcarbamimidoyl ammonium carbonate hydrate’s properties for new roles in green chemistry as a recyclable base or bifunctional catalyst. Material scientists see opportunity in doping it into functional polymers or next-generation adhesives, aiming for smarter molecular switches. Drug design may pick up pace as researchers unlock new biological targets requiring stable, easily derivatized guanidine pharmacophores. The push towards sustainable, less hazardous chemistry urges researchers to rethink solvent, waste, and process choices, and this product’s water-compatibility puts it ahead of many legacy compounds. The landscape changes as old rules meet the demands of precision science, and every incremental improvement brings real benefits—better safety, reduced cost, and smarter innovation.
Walk into any busy research lab and you’ll see shelves lined with bottles labeled with names long enough to break a game of Scrabble. One of these, (N-Phenylcarbamimidoyl)Ammonium Carbonate Hydrate, sounds mysterious but has a solid place in synthetic chemistry and pharmaceutical research. If you ask a synthetic chemist about it, chances are they recognize it as a specialized reagent, not something just anyone keeps on hand. The work I’ve seen done with it focuses on modifying molecules, and that matters a whole lot when developing new medicines or materials. The world of organic synthesis thrives on building and tweaking carbon-rich compounds, and this ingredient steps in when researchers need to form bonds in specific ways—especially with introducing guanidine groups to molecules.
Drug research depends on making small tweaks to molecular structures. By introducing a guanidine group (that’s a big family in biochemistry), scientists can “tune” how a drug acts in the body or how well it latches onto a particular protein. This can mean the difference between a pill that works and one that gets flushed out before it can help. (N-Phenylcarbamimidoyl)Ammonium Carbonate Hydrate is no household name, but the impact it has on producing potential medicines deserves attention. In my time around industry professionals, I learned researchers treat such reagents as part of their toolkit for inventing novel compounds when nature needs a push.
Beyond drugs, the chemistry here finds use in creating “tags” or markers for biological testing. Diagnostic companies constantly search for better ways to detect diseases or track molecules in complex systems like blood or cells. To pull this off, they engineer chemical markers—some rely on guanidine chemistry, with this reagent providing a solid route to attach the right groups. Deep in the world of protein-protein interactions and enzyme research, attaching these groups changes how molecules behave in real-world conditions, making samples easier to read.
Experience has shown me that specialty reagents always come with handling challenges. Any chemical with complex names and “hydrate” in the label loves water and doesn’t mind turning unstable if stored wrong. Good labs pay close attention to safety when they open such bottles: gloves, goggles, solid ventilation, all the usual gear. The material safety data for this compound gives crystal-clear instructions—not just to keep folks safe but to make sure the chemistry doesn’t get ruined by stray moisture or air. Without these precautions, the best research hits a wall.
In the United States and across Europe, researchers and suppliers follow regulatory standards like those from the FDA or EMA. Reagents that help develop pharmaceuticals have to be traceable and pure, since any problem at this basic level risks entire research projects or, further down the road, patient safety. From my own projects and others I’ve observed, strict documentation and supplier vetting come into play every day. Companies don’t just buy chemicals—they review certificates of analysis, question suppliers, and test purity in-house. This attention keeps experiments reliable and protects future patients.
Pharmaceutical and biotech teams always push for tools that let them make molecules faster, safer, and at lower cost. Compounds like (N-Phenylcarbamimidoyl)Ammonium Carbonate Hydrate give scientists leverage for building up chemical complexity, sometimes shaving months off development timelines. Industry partnerships with chemical suppliers also now focus on green chemistry—safer solvents, better waste handling, and less environmental impact. For me and anyone who’s watched new drugs work their way from benchtop to bedside, seeing progress in safer, smarter reagents isn’t just good business, it’s good science.
Chemical names can get long and intimidating, pulling together bits from different functional groups. In the case of (N-Phenylcarbamimidoyl)ammonium carbonate hydrate, the name carries a lot of clues. "N-Phenylcarbamimidoyl" points to a guanidinium group linked to a phenyl ring. "Ammonium carbonate" brings together ammonia and carbonic acid. "Hydrate" means that water molecules tag along in the crystal structure. Scratching beneath the surface, all these fragments sum up to form a single chemical entity.
Piecing it together isn't just a routine puzzle. Experience tells me most mistakes happen where functional groups overlap or, sometimes, extra water goes missing. Let's tackle each part:
The core formula for (N-Phenylcarbamimidoyl)ammonium is C7H9N3+. Adding carbonate means two of these cations balance a single carbonate anion: (C7H9N3)2CO3. Introducing hydration, the typical hydrate gets a single water molecule: (C7H9N3)2CO3·H2O.
Every time I walk into a lab and see a new compound, my first move is to check that formula. Incorrect formulas don’t just lead to confusion—they turn syntheses into costly guessing games. Mistakes can ripple into quality issues, especially across pharmaceutical and agricultural products. Regulations like those enforced by REACH in the EU or OSHA in the U.S. push for strict accuracy on material safety data sheets. An error could even violate the law.
Even outside the lab, precision in reporting formulas affects safety in transport, storage, and application. The wrong water content, for instance, shifts molecular weights, which can mean disaster in formulation. Misreporting ammonium content might signal a completely different reactivity or hazard profile.
Data from databases like PubChem and ChemSpider support the hydrated formula (C7H9N3)2CO3·H2O as correct. Cross-checking manufacturer datasheets never hurts either. If more waters of hydration appear in the product, the suffix simply changes: ·2H2O, ·3H2O, and so on, but the backbone stays the same.
Anyone working around chemicals owes it to themselves and others to double-check raw formulas. Lessons from old accidents show that a mix-up can lead to fires, toxic vapors, or environmental harm. Even small slip-ups—like missing a water molecule in a hydrate, or confusing carbonate with bicarbonate—have made news, sometimes with tragic endings.
Every good chemist gets into the habit of confirming formulas before use. Researchers who set the example help keep their labs safe and their papers respected. One fix: maintaining access to up-to-date chemical registries. Another: building peer review into protocol, so at least two pairs of eyes cross-check details before anything goes out the door. It's not just about ticking regulatory boxes—it's about upholding a commitment to real-world safety and making sure the next person in line inherits facts they can trust.
Dealing with chemicals like (N-Phenylcarbamimidoyl)Ammonium Carbonate Hydrate brings real concerns. This compound, used in research and specialty synthesis, isn’t just another powder on a shelf. Its reactivity, moisture sensitivity, and unknown toxicological profile should give anyone pause before careless storage. I’ve walked into too many labs where powders—sometimes as unpredictable as this one—were stored next to open wash bottles or half-screwed jars. What sits on the shelf today might react into something dangerous tomorrow if no one pays attention.
Heat, water, and incompatible substances can spell trouble. Some folks shrug off storage guidelines, thinking those rules only matter for high-volume chemicals. My experience disagrees. A few grams in the wrong spot can create a mess or worse, send someone to the emergency room. Responsible storage isn’t about paranoia; it’s about respecting risk, even in low quantities.
I remember one chemist who kept all his reagents in a cabinet under a sink. The area was damp—no surprise, a slow leak dripped behind the bottles. When a humidity-sensitive compound broke down, the rotten-egg stench filled the building. He learned quickly that some compounds need protection from moisture and heat—two things a leaky pipe delivers.
For this compound, the first enemy is moisture. I always recommend using air-tight bottles with sturdy seals. After opening, toss in a fresh desiccant packet and check frequently for clumping or discoloration. A desiccator cabinet or dry box saves time and prevents many headaches. Storing in a cool, shaded spot, far from any heat source, keeps degradation in check. Temperature swings in makeshift shelving seem harmless but accelerate breakdown and contamination more than most realize.
Direct sunlight isn’t just bad for human skin. Many organic compounds, including this one, fall apart under UV exposure. I’ve worked in places where reagents sat in clear bottles on windowsills. A few months later, not only were labels faded, but contents had gone off-color and unusable. Store chemicals away from windows. Opaque containers or cabinets close that loophole for light damage.
Experience in research settings has shown that clear, thorough labeling makes a difference. Sometimes, a bottle passed through five hands before landing with me. Labels included dates opened and moisture warnings: a small act that saved hours and probably avoided a few disasters.
Grouping incompatible chemicals risks dangerous reactions, contaminations, or worse outcomes. This compound shouldn’t share a cabinet with acids, bases, strong oxidizers, or even casual “overflow” bottles. Dedicated shelves for different chemical families keep things orderly and safe, especially in small spaces.
Storeroom orientation is a must for anyone new. Real respect for lab safety comes from stories, experience, and checking up on guidelines. It isn’t about following rules for the sake of bureaucracy. It’s about getting home safe and keeping everyone in the building breathing easy.
Research on this compound’s long-term health impact remains thin. Until more is known, gloves, goggles, and caution belong in every step—from receipt to disposal. My own rule: treat every unfamiliar chemical as if it’s worse than you think, even if handling it feels routine.
I’ve seen plenty of clever tricks for storage—vacuum-sealing, secondary containers, and digital inventory tracking. The best solution always started with respect for the hazards. Good storage isn’t complicated or expensive, just smart and preventative.
Simple steps matter most. Dry, cool, dark. Proper seals. Clear labeling. Smart segregation. Reminders shared with every new colleague. Safety builds from these basics, every single day.Working with any chemical that sounds like a tongue-twister usually shows up with its own set of headaches. (N-Phenylcarbamimidoyl)Ammonium Carbonate Hydrate brings its own flavor of potential hazards. This compound doesn’t win awards for volatility, but it still demands care. Left unchecked, people can run into trouble with skin or eye contact, inhalation, or accidental ingestion.
Nobody should have to learn the hard way that a splash of unidentified powder burns for hours. Gloves, goggles, and a lab coat stand as line one of defense—real lifesavers, no matter how seasoned you are. The lab sometimes feels like home, but it’s not the kitchen. Splashes that look like a minor mess can mean hours flushing your eyes at the sink. Proper protective gear cuts down your risk by a mile.
Dry powders like this one kick up dust, and that dust shouldn’t end up in your lungs. Fume hoods or strong exhaust fans belong in every space that hosts this chemical. During my years in labs, I’ve seen people brush off a slight smell or that “harmless” white mist as no big deal. That little cloud can irritate your nose, lungs, and even eyes. Setting up proper ventilation only costs a few minutes but pays dividends in health later down the road.
Over the past decade, I’ve watched new researchers dip fingers directly into powders or liquids. They almost always regret it. Even a brief handshake between palm and chemical can produce redness, rashes, and other reactions. Always wear gloves—but more importantly, use the right ones. Nitrile works for most chemicals, but check your safety datasheet before putting on just any pair. Face shields and chemical splash goggles beat regular reading glasses any day.
Spills sound small, but they force everyone to stop work and switch gears. Swift response starts with knowing where to find spill kits, neutralizers, and an emergency shower. Don’t sweep up with bare hands; use dedicated scoops and keep the area well-marked to warn others. This habit grows out of real events—a poorly cleaned spill can drag a simple workday into an emergency hospital trip.
Drawers and shelves cluttered with open containers just invite mistakes. This chemical wants a sealed container, tucked away in a dry, cool place. Label everything with the date, the exact name, and any warnings you can think to add. Once in a while, someone finds an unmarked jar and wonders what’s inside. Proper labeling means fewer surprises.
Tossing leftover powder in a regular trash can tempts fate. Follow your lab’s hazardous waste policy—spill it into the right container, close the lid, and get it out of working spaces. It’s easier to fill out paperwork and make quick pickups than to call a cleanup crew for an office accident.
Working alone with complicated substances breeds trouble. Make sure everyone on your team reviews safety protocols before starting a project. Share those “almost” accidents so the next person doesn’t repeat the same mistakes. A well-practiced team spots hazards and fixes them before they blow up.
Chemicals look harmless on a data sheet, but real-world lab experience comes with a healthy distrust of anything without clear specs. In my time running reactions, a compound’s purity changed everything: clean glassware, reliable yields, and safer handling. With (N-Phenylcarbamimidoyl)Ammonium Carbonate Hydrate, small impurities cloud structural analysis and throw off syntheses that run on tight margins. Even trace contamination brings unwanted byproducts that feel like a small leak in a big process—easy to ignore, frustrating to fix down the line.
Suppliers sell this compound with grades meant for different users: research, technical, or pharma. At the research level, 95% might look tempting for screening projects. When scaling up or supporting pharma development, tighter specs matter. A reputable supplier will test for heavy metals, moisture content, and common organic impurities. Relying only on the chemical’s label is a shortcut to uncertainty. I’ve found that batch certificates with proper chromatograms back up a supplier’s claim better than generic descriptions. The best labs reject lots that don’t come with this documentation.
I’ve stood in a lab, reading grainy supplier datasheets, knowing one typo could hold up months of work. That feeling gets worse without a clear purity profile and grade breakdown. Once, a production run stalled for days because a supposed “analytical grade” chemical still held trace dyes, invisible to the eye but immediately flagged in LCMS. Those who rely on suppliers without transparency get caught in a guessing game that is expensive, slow, and always stressful.
The best suppliers follow ISO certification or cGMP protocols, so purity statements have real backing. Labs with a history of FDA inspections can’t afford slip-ups. Details on heavy metals, residual solvents, and microbial contamination carry extra weight in regulated fields. Scientists can’t skirt these details—government agencies conduct random spot checks, and a single contaminant holds up batch releases. Relying on vendors that maintain batch traceability, provide full spectra, and update safety data sheets regularly reduces anxiety across the production and quality assurance chain.
Before purchasing, request batch-specific certificates and actual impurity profiles. Ask about their purification steps and history of addressing non-conforming batches. Some smart labs conduct rapid in-house purity checks even after delivery. Contracts with suppliers should build in penalties for mislabelled or out-of-spec batches; this protects timelines and budgets. Teams benefit most from an open chain of communication—having direct access to a supplier chemist prevents a lot of the confusion down the line.
Labs on a budget sometimes cut corners, but past experience reminds me that upfront savings often cost more in wasted effort and spoiled batches. Knowing the true grade and purity of your (N-Phenylcarbamimidoyl)Ammonium Carbonate Hydrate means fewer failed experiments, more reliable results, and a smoother path from the benchtop to production scale.
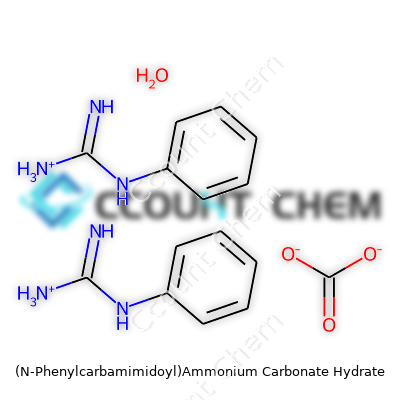
| Names | |
| Preferred IUPAC name | Ammonium (Z)-N-phenylcarbamimidate carbonate hydrate |
| Other names |
Carboxybiuret Ammonium Phenylhydrazone Ammonium phenylcarbamimidate carbonate hydrate Phenylguanidine carbonate hydrate |
| Pronunciation | /ɛn ˈfiːnɪlˌkɑːrbəˌmɪdɪˌɔɪl æˈmɒniəm ˈkɑːrbəneɪt ˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 94158-13-1 |
| Beilstein Reference | 2931295 |
| ChEBI | CHEBI:145262 |
| ChEMBL | CHEMBL4251979 |
| ChemSpider | 32815888 |
| DrugBank | DB11360 |
| ECHA InfoCard | 03c8b714-a147-4ffb-80b8-7c534f2b488a |
| EC Number | 68828-81-7 |
| Gmelin Reference | 1565287 |
| KEGG | C18647 |
| MeSH | D013494 |
| PubChem CID | 162143163 |
| RTECS number | BV9810000 |
| UNII | W9111J8R5W |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H15N5O4 |
| Molar mass | 292.28 g/mol |
| Appearance | white solid |
| Odor | Odorless |
| Density | 1.41 g/cm3 |
| Solubility in water | Soluble |
| log P | -2.7 |
| Acidity (pKa) | 13.7 |
| Basicity (pKb) | 2.9 |
| Refractive index (nD) | 1.649 |
| Dipole moment | 4.1914 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.7 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | B06AC01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P330, P337+P313, P501 |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 820 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.05 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Guanidine Phenylguanidine Ammonium carbonate Carbamide (urea) N-Phenylguanidine hydrochloride |