Taurine derivatives have drawn interest ever since researchers finished charting the unique roles of simple sulfur-containing compounds in biological systems. Early investigations into amino sulfonic acids and their reactions with carbamoyl agents opened the doors. Chemists in Europe worked through the mid-20th century searching for taurine analogues with novel hydration or buffering functions. Processing taurine with carbamoyl chloride delivered N-(Carbamoylmethyl)taurine, a molecule with properties noticeably different from its parent. Academic curiosity and commercial pressures joined forces. Consumer awareness of taurine in energy drinks, nutrition, and medicine fed more funding into studying its derivatives. Most of the critical patents and synthetic techniques cropped up in the 1970s and 1980s, as chemical companies sought safer surfactants and new biomedical building blocks. Several decades of refinement have brought efficiencies in preparation and greater insight about physiological tolerances. As of the present, the molecule stands out among taurine derivatives for its blend of chemical stability and adaptability in research.
N-(Carbamoylmethyl)taurine, often called CMTAU in industrial circles, exists as a white to off-white crystalline solid in its pure form. Researchers tend to keep it in high-purity, sealed containers, as moisture and oxidizing agents slowly degrade the compound over months. Marketed under a handful of specialized chemical trade names—including CMTAU, Taurylurea, and Urea N-Taurine—this compound regularly turns up in research catalogs and custom synthesis listings. Formulators in biochemistry or pharmaceutical labs rely on its solubility in water and simple handling at ambient temperatures. End-users pay attention to batch purities, because even minor impurities can interfere with sensitive biological applications. Product datasheets routinely outline heavy metal content, traces of unreacted taurine, and any antimicrobial residues from preparation.
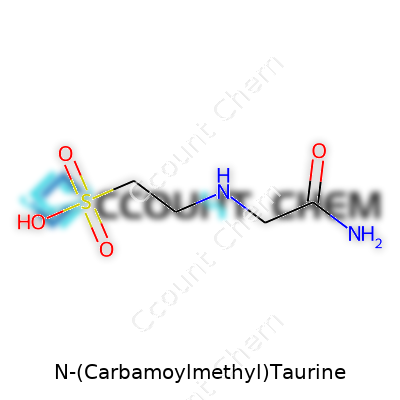
N-(Carbamoylmethyl)taurine checks in with a molecular weight of 195.21 g/mol. It dissolves well in deionized water but resists most organic solvents. The melting point floats between 204–208°C—a sign of strong intramolecular hydrogen bonding. It produces a faint ammoniacal odor at elevated temperatures, mostly from slow thermal decomposition. Chemically, this compound features a primary sulfonic acid group, a carbamoyl-amino linkage, and a two-carbon backbone. The sulfonic acid group keeps it highly polar and increases its compatibility with physiological systems. The compound maintains a neutral to slightly acidic pH in water, depending on buffer conditions. Its chemical stability holds up during months of room-temperature storage, as long as it stays sealed from atmospheric humidity.
Vendors highlight the importance of accurate labeling for N-(Carbamoylmethyl)taurine, noting full chemical name, synonyms, batch number, date of manufacture, stated purity (commonly 98% or higher), and trace analytical results (such as sulfate, ammonium, and heavy metal levels). Hazard pictograms are required, alongside safe-handling standards, even though toxicity falls below many common reagents. Often, packs ship in amber vials or polyethylene bags to block UV degradation, using inert atmosphere fills. Laboratories maintain careful logs of storage temperature, opening dates, and resealing status, since cross-contamination or exposure can reduce assay dependability. Technical sheets sketch out suggested handling concentrations, solubility limits, and shelf life, always stressing that researchers confirm compatibility with sensitive cell cultures, tissue models, or analytical instruments before adoption.
The key synthetic route starts with taurine, a plentiful commodity for laboratory synthesis. Carbamoylation proceeds by reacting taurine with urea or a suitable carbamoyl donor in aqueous or methanolic solution, often with base catalysis and moderate heating. Typical yields average between 70% and 85% on small to medium lab scale. Workup involves precipitation with acetone or ethanol, followed by filtration and drying under vacuum. Scale-up brings new challenges, as higher temperatures and longer reaction times can drive by-product formation, so companies periodically improve their standard operating procedures for cleaner conversions. Process control has driven down costs and environmental impact, moving away from toxic solvents and expensive catalysts. Environmental regulations in Europe and North America have forced facilities to treat spent process water for trace taurine and urea contamination.
N-(Carbamoylmethyl)taurine remains stable under neutral aqueous conditions, but strong acids or bases cleave the carbamoyl group back to taurine and urea derivatives. Oxidizing agents bring further transformation, sometimes generating sulfonic acid cleavage products that lose biological relevance. Chemists tune the reactivity by adding alkylating or acylating agents to its nitrogen atoms, sometimes making it more hydrophobic or reactive toward peptide synthesis. Bioconjugate chemists leverage this molecule as a linker, because the carbamoyl group can participate in amide bond formation without triggering unwanted side reactions. Newer research investigates using the taurylurea scaffold to anchor fluorescent dyes, affinity tags, or small-molecule drug candidates, banking on its solubility and lack of background interference with analytical readouts.
Researchers refer to the compound as Taurylurea, CMTAU, N-Carbamoylmethyltaurine, and Urea N-Taurine, among other synonyms. Catalog suppliers sometimes list product codes, such as CAS 53329-26-7, or proprietary trade names for registry simplicity. Practitioners emphasize proper identification in research notes and publications—confusing CMTAU with N-acetyltaurine or other taurine derivatives leads to dosing errors or the wrong choice in biological testing. The shift in naming generally tracks with regional sourcing or institutional preference. Synonyms need to remain clear, ensuring safety protocols and supply chains keep in step.
Safety with N-(Carbamoylmethyl)taurine focuses on basic chemical hygiene. Inhalation risks remain low at standard laboratory concentrations, but ingestion or contact with eyes can cause mild irritation. Proper ventilation, glove use, and protective eyewear form the backbone of safe handling. Disposal recommendations include neutralization and controlled sewage release within site-specific limits, following local chemical waste rules. Industrial hygiene teams monitor for airborne dust, especially during bulk weighing and packing. Technicians implement spill kits and eyewash stations as daily safeguards. Material Safety Data Sheets accompany each shipment to document risks, storage needs, and first-aid directions. Personal accounts in research labs point to the value of keeping closed waste containers and avoiding food or drink near preparation areas, even for low-toxicity compounds like CMTAU.
N-(Carbamoylmethyl)taurine finds work as an intermediate for drug synthesis, a functional additive in cosmetics, and a component of customized hydrating solutions. Biochemical researchers often use it in studies on membrane transporters or to probe taurine metabolism pathways. Its unique blend of solubility and mild chemical reactivity has inspired trials as a stabilizer for proteins or peptides in solution. Some synthesis clusters leverage the molecule as a mild buffer or to mediate crosslinking reactions in polymer science. In animal nutrition, additive testing explores whether taurylurea enhances absorption of specific micronutrients, given taurine’s known role in bile salt conjugation. Diverse roles pop up in patents for surfactant modification, tissue engineering scaffolds, and food technology.
Recent years brought fresh curiosity to taurine and its derivatives, as scientists sort out how changing the carbon and nitrogen skeleton shapes physiological response. Research groups worldwide publish findings on CMTAU’s impact on cell growth, osmotic balance in mammals, and as an antioxidant in aging studies. Private sector R&D investigates improved synthetic routes and purification strategies, aiming to minimize residual impurities and unreacted starting materials. At least two industrial consortia have pushed for green chemistry standards in CMTAU production, reducing water use and eliminating halogenated solvents. Future work looks at combinatorial libraries using N-(Carbamoylmethyl)taurine as a chemical handle to discover small-molecule therapeutics, especially as high-throughput screening platforms favor water-soluble candidates.
Toxicology on N-(Carbamoylmethyl)taurine points to a generally low biological hazard, with acute oral and dermal LD50 values well above concentrations found in research use. Subchronic studies in rodents failed to find changes in core organ function or behavior at relevant dosing. Some in vitro work checked CMTAU’s impact on human liver and kidney cell lines, observing minimal cytotoxicity up to moderate concentrations. Regulatory agencies classify it as non-hazardous in laboratory contexts, though caution remains for vulnerable populations and unknown chronic exposures. Researchers keep pressing for long-term metabolite analysis in mammals, mapping out pathways to clarify if any downstream effects escape current detection. So far, most authorities treat it as roughly as safe as taurine itself, once proper chemical hygiene is followed.
Looking ahead, N-(Carbamoylmethyl)taurine’s fate sits tied to the broader appreciation for designer biomolecules in pharmaceutical development, food applications, and advanced materials. More companies see value in water-soluble, low-toxicity intermediates that can simplify synthesis and reduce process waste. University scientists dig into structure–activity relationships to tease out which modifications might yield new drugs, metabolic probes, or tissue engineering scaffolds. Environmental sustainability shapes how bulk chemical facilities approach scaling up production. Advances in green chemistry and biocatalytic methods hold promise for cleaner, safer preparation. CMTAU bridges the gap between commodity chemicals and specialty molecules, offering a foundation for the next wave of functional material and biomedical innovation.
N-(Carbamoylmethyl)Taurine seems like a mouthful, but the work it handles behind the scenes in chemistry and biology packs real impact. This compound emerges most often as a product made by tweaking taurine with a carbamoylmethyl group. Taurine rings a bell for many because of its role in energy drinks and its value in animal nutrition. Tweaks like these carve out new uses that often shape clean water, medical progress, and even agriculture.
You’ll come across N-(Carbamoylmethyl)Taurine in places where water needs to stay clean, especially in household and industrial scenarios. Here, this chemical blends into a class known as zwitterionic surfactants. These molecules help scrub stubborn dirt, break up oil, and make rinsing a breeze. Surfactants built on taurine keep foaming low and work well even if the water holds calcium or magnesium. For those who run laundry businesses or use hard tap water, the impact shows up as softer towels and clearer dishware. Research backs up the cost savings and waste reduction that occur when surfactants like this step in, and that matters for both business and the environment.
Taurine-based compounds carry a reputation for being kind to living tissues. This makes N-(Carbamoylmethyl)Taurine and similar compounds favorites for drug researchers. They often act as intermediates or building blocks in making certain drugs easier for the body to absorb. That links up with my experience in biochemistry labs, where we constantly look for compounds that balance potency with safety. Pharmaceutical teams look for ways to wrap medication molecules in something that keeps damage to healthy cells down, and taurine derivatives step up for this task. With the global push for more accessible medicines, small tweaks like adding a carbamoylmethyl group can help reach the balance between effect and safety.
Athletes and pet owners might know taurine as a supplement, but chemists combine it with groups like carbamoylmethyl to stretch its functions inside the body. There’s ongoing research into how these taurine-based compounds affect metabolism and mineral transport in animals. Scientists track improvements in gut health for livestock or better nutrient uptake, making this chemical a quiet support player in the food supply chain. In the grand scheme, that can slide into savings for farmers and, in turn, more stable food prices for the average shopper.
While the positives stand out, scaling up production safely always sits on my mind. Factories that make N-(Carbamoylmethyl)Taurine must handle raw materials responsibly to limit waste and chemical spills. Regulatory checks help, but the best change comes from investing in cleaner reactors and recycling solvents. Any chemist who’s dealt with caustic by-products will agree that swapping out harsher agents for greener alternatives can save headaches and keep neighborhoods safer. Working with local universities or tapping into public research grants also builds better pathways for sustainable chemical production.
Everyday life rarely puts a spotlight on compounds like N-(Carbamoylmethyl)Taurine, but the benefits show up where people care about clean water, safe medications, and affordable food. Supporting smart research, safer factories, and fair regulations keeps society moving forward, rooted in respect for science and what it brings to the table.
Stepping into the world of food additives often means sorting through chemical names you never thought you’d hear at the dinner table. N-(Carbamoylmethyl)Taurine has been popping up more lately. It’s a compound getting attention for potential uses in supplements and specialized diets. Anyone hearing its name for the first time will ask if it’s safe.
Taurine itself rings more of a bell. It's found naturally in the body and lots of people recognize it from energy drinks. Carbamoylmethyl groups get tacked onto all sorts of molecules during pharmaceutical development or food processing, creating new variants that sometimes work differently. Toss those two ideas together and you get N-(Carbamoylmethyl)Taurine, a synthetic molecule aimed at supporting metabolic processes.
I’ve seen the habit of scanning labels for ingredients I can pronounce. That habit pays off most with chemicals that’re still new to consumers, and this compound is definitely on that list. Most safety questions boil down to a simple search: Are there peer-reviewed studies showing this additive’s effect on living cells, organs, or human health? For N-(Carbamoylmethyl)Taurine, studies in test tubes or on rats can show what to expect, but nothing replaces research carried out on people eating normal amounts through a typical diet.
So far, most published data focuses on how this compound behaves in laboratory assays, with a little bit of animal research sprinkled in. Safety claims sometimes get ahead of the research, which makes careful reading essential. Agencies like the FDA or EFSA usually won’t give a green light on a new food ingredient until researchers run repeated, in-depth trials—even more so for synthetic compounds. Neither agency has listed N-(Carbamoylmethyl)Taurine as generally recognized as safe (GRAS), according to their current databases as of this year.
This isn’t about fearmongering—taurine alone has a solid track record in food science. Still, changing a small part of a molecule can make a big difference in its safety profile. For example, trans fats started out as a simple twist on vegetable oil, but turned out to have very real risks that were only understood decades later. A synthetic modification needs proof that it doesn't build up in the body, interact with common medications, or act differently in people living with chronic conditions. Testing methods like in vitro studies and animal models help flag some risks, but individual people process chemicals in surprising ways.
No substitute exists for direct long-term studies in humans. Full transparency on sources, manufacturing processes, and intended use matters. A good practice is always to publish safety data openly, letting outside researchers dig deeper. Gaining approval from food safety authorities requires independent studies confirming its safety over months or years, not days or weeks. Companies with new compounds on the market should fund large randomized controlled trials and put warnings on labels about unknown risks.
For now, consumers and food companies should lean toward caution. I keep an eye out for transparent product labels, clear information from supplement manufacturers, and honest statements about the state of research. There’s nothing wrong with pausing before putting a brand-new additive into your body until research catches up. This approach builds trust in food science and helps keep meals as safe as possible. If future studies give N-(Carbamoylmethyl)Taurine a clean bill of health, that will mean the research did its job. If not, there will be proof strong enough to guide smarter choices.
N-(Carbamoylmethyl)Taurine presents itself as a small organic molecule featuring both sulfonic acid and amide functional groups. Breaking down the name gives a pretty strong sense of its behavior. Taurine on its own stands out for being a sulfonic acid, not a carboxylic acid like those in amino acids. Fusing that with a carbamoylmethyl moiety—a simple amide—gives this compound a mix of properties. Sulfonic acids stay highly polar, strongly acidic, and attract water. The amide slipstreams in with different handling, standing stable and slightly less reactive, but able to form hydrogen bonds. This combo brings flexibility to the molecule’s solubility and reactivity in aqueous solutions.
Most everyday experiences with chemicals that have sulfonic acid groups involve high water solubility and resistance to breakdown under harsh pH swings. N-(Carbamoylmethyl)Taurine remains strong in water because of the taurine backbone, and the molecule handles both acidic and basic conditions without falling apart easily. Sulfonic acids don’t decompose quickly, unlike esters or some amines. The amide part brings a bit more structure but doesn’t throw the molecule off its game. Because amides as a class need serious force (think strong acids or bases, heat, or enzymes) to react in a big way, N-(Carbamoylmethyl)Taurine stays calm during most reactions. This makes the molecule handy in settings that need a dependable, non-reactive base for further chemistry.
Living with chemistry at home or in the lab, I see searchable differences once you combine sulfonic acids and amides. The sulfonic end loves water, which helps N-(Carbamoylmethyl)Taurine dissolve quickly, much like taurine-based products in sports drinks or supplements. The amide portion adds just enough “grip” for hydrogen bonds, anchoring to proteins or enzymes if needed. In practice, such a structure opens up a world of compatibility—mixing easily into formulas, working around metals without sparking unwanted reactions, and staying non-volatile even under heat.
Sulfonic acids can help with binding metals, cleaning, or buffering in lab settings. N-(Carbamoylmethyl)Taurine’s stable profile keeps it safe to handle, even when mixing with strong acids or bases. I appreciate having a chemical like this on the shelf, since it doesn’t react with most household or laboratory agents unless conditions are extreme. Researchers have looked at taurine derivatives for medical uses, like supporting kidney function or acting as a mild chelating agent in dialysis. The extra carbamoyl group may open doors to better stability or more fine-tuned interactions in the body, without making the compound too aggressive or easily broken down.
Handling sulfonic acid derivatives comes with an expectation of low toxicity and minimal smell. Amides, especially small ones, usually share this good reputation. N-(Carbamoylmethyl)Taurine likely follows suit, posing few risks outside the basic eye or skin irritation you’d expect from contact with any strong acid or base. In water, rapid dissolution means quick dispersal, reducing chances for dangerous buildup.
Stepping away from abstract terms, I value N-(Carbamoylmethyl)Taurine as a stable, water-loving molecule with gentle reactivity. The mix of sulfonic acid and amide groups hands over a balance of strength and safety. For chemists, formulators, or anyone facing water-based challenges in their work, this molecule doesn’t just show up for one specialized job—it fits into many, from lab benches to medical settings.
I've seen how storage mistakes lead to all kinds of headaches, from ruined samples to dangerous situations in the lab. Experienced researchers and newcomers know that safe handling starts from the very moment chemicals arrive. N-(Carbamoylmethyl)Taurine, not a household name for most people, crops up in specialized projects in biochemistry and analytical work. It's not the most hazardous compound in the lab, but careless storage can still cause trouble for your data, equipment, and the people working alongside you.
The general rule with organic compounds, especially those containing both amide and sulfonic acid groups, is to keep them away from heat sources and avoid big swings in temperature. Storing N-(Carbamoylmethyl)Taurine at room temperature, ideally in a dry and well-ventilated location, prevents degradation and reduces the risk of unexpected reactions. Too much moisture or sunlight can trigger breakdowns over time, leaving you with inconsistent results or mystery contaminants.
I've worked in labs where a poorly sealed bottle was left next to a sunny window, and that mistake cost us weeks of research. Light and warm temperatures give energy to all sorts of unwanted side reactions, and sensitive compounds like this one lose purity or even start producing odors no one wants to deal with. A cool, dark cupboard far from heat-emitting equipment goes a long way. Most modern chemical storage rooms already manage temperature and airflow for safety.
There’s a reason most suppliers package chemicals with desiccants. Opening a jar in a humid room lets water vapor in and that raises the risk of caking or even mild hydrolysis. I always keep these compounds in tightly sealed containers, and if possible, toss in a fresh silica gel packet. For smaller quantities, a desiccator cabinet keeps everything dry and stable. Moisture doesn’t just mess with the chemistry—it can make weighing samples a nightmare.
In shared spaces, clear labeling keeps everyone safe and cuts down confusion. Someone mixing up white powders or misreading a faded label causes more accidents than anyone wants to admit. Mark the date received and date opened. Use the manufacturer’s original container if you can, since they’ve already done the work to ensure chemical compatibility.
Separating chemicals by hazard class and storage requirements prevents cross-contamination. N-(Carbamoylmethyl)Taurine should stay away from acids, oxidizers, and strong bases, even if you only notice mixing issues in rare situations. Taking the extra thirty seconds to return a bottle to the right shelf pays off in fewer headaches later.
Ordering only what you can use in a reasonable timeframe is one of the most overlooked aspects of good storage. Old chemicals—especially those opened and closed a dozen times—accumulate more risk every year they sit on the shelf. Keeping a regular inventory helps you track what’s getting stale or low and gives you a chance to discard out-of-date materials before they cause problems.
Proper storage of anything, including N-(Carbamoylmethyl)Taurine, doesn’t just protect your work—it shows respect for your team, the facility, and the people who handle disposal further down the line. Good habits spread. The simplest storage solutions—cool, dry, dark, sealed—stay reliable year after year, saving time and building trust in every result you get.
Every so often, a chemical compound draws the attention of buyers outside the typical laboratory supply crowd. N-(Carbamoylmethyl)Taurine has popped up on radar screens of scientists, industry professionals, and curious buyers. Folks who want this compound usually work with it for research on amino acid derivatives or specialty surfactants. My background in chemistry labs has taught me this: working with chemicals such as this one takes more than a shopping cart and a credit card. Safety, supply reliability, and legitimate use sit high on the list.
Stepping into the realm of chemical purchasing, there’s little room for error. Most reputable suppliers want to check out your credentials, be they a research institution, medical lab, or industrial user. I’ve had to go through identity verifications and submit project details before companies approved my orders for less common molecules.
The simple truth: this isn’t something you’ll spot sitting on e-commerce sites like Amazon. Instead, buyers rely on chemical supply specialists like Sigma-Aldrich, Alfa Aesar, or TCI Chemicals. These companies openly publish their catalogs and provide safety data sheets, sometimes along with pricing, pure enough for laboratories. A quick search for N-(Carbamoylmethyl)Taurine on their platforms can pull up what’s available and in what quantity and purity.
Global regulations can tangle up sourcing. Some chemical suppliers won’t ship to certain countries without a slew of paperwork—from end user declarations to import licenses. Anyone hunting for N-(Carbamoylmethyl)Taurine ought to check their country’s rules. Last year, when our lab group tried bringing in a rare precursor for an amino sulfonate, shipping took two months longer due to licensing slowdowns. A supplier rep told us they see these hang-ups with a lot of specialty compounds.
Beyond red tape, pricing bites hard. Low-demand compounds have high production costs because they’re not made in bulk. Quotes for specialty biochemicals often sound extreme, especially for small research teams and start-ups. Students and citizen scientists face even steeper odds without institutional accounts.
As someone who’s seen corners cut in pursuit of rare chemicals, picking a trustworthy supplier matters. Look for businesses with ISO certifications or similar standards. Read customer reviews and ask for recent certificates of analysis—the last batch I sourced came with HPLC and NMR data. Avoid open-exchange online marketplaces where sellers can fade away after a sale, and push back if anyone is slow to show essential paperwork.
Chemical supply chains have changed a lot in the past decade. Some smaller firms have popped up to serve niche research groups. These newer players sometimes offer lower minimum order quantities and more responsive customer service. Even so, the legal and ethical responsibilities stay the same. Questions about legitimate use, environmental handling, and user safety should never be skipped.
Better transparency in supply chains would take some guesswork out of sourcing. Standardized online verification for buyers could also speed up the process for professionals without sacrificing oversight or safety. For folks operating outside of big institutions, more community-driven directories of vetted suppliers could help. Until then, patience and respect for regulations drive the process. Only buy from trusted sources, and keep the focus on responsible use—anything less can backfire on everyone involved.
| Names | |
| Preferred IUPAC name | 2-[(2-Sulfanylethyl)carbamoyl]acetamide |
| Other names |
N-(Carboxymethyl)taurine N-(2-Sulfoethyl)glycine |
| Pronunciation | /ɛn kɑːˈbæmɔɪlˌmɛθɪl ˈtɔːriːn/ |
| Identifiers | |
| CAS Number | 7432-24-0 |
| 3D model (JSmol) | `3D structure; JSmol; Mol* viewer; O=C(NCCS(=O)(=O)O)N` |
| Beilstein Reference | 609371 |
| ChEBI | CHEBI:140348 |
| ChEMBL | CHEMBL2086617 |
| ChemSpider | 13382120 |
| DrugBank | DB08357 |
| ECHA InfoCard | 03a274ae-bbff-4624-bfd3-f17e427cac3c |
| EC Number | 3.5.1.79 |
| Gmelin Reference | 107142 |
| KEGG | C21154 |
| MeSH | D020123 |
| PubChem CID | 107728 |
| RTECS number | GN8490000 |
| UNII | 2026B3F62M |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID4021356 |
| Properties | |
| Chemical formula | C5H10N2O4S |
| Molar mass | 209.23 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.54 g/cm3 |
| Solubility in water | soluble |
| log P | -2.69 |
| Acidity (pKa) | -1.63 |
| Basicity (pKb) | 1.01 |
| Magnetic susceptibility (χ) | -51.4×10^-6 cm^3/mol |
| Dipole moment | 4.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | N-(Carbamoylmethyl)Taurine |
| Pharmacology | |
| ATC code | N05CM24 |
| Hazards | |
| Main hazards | May cause skin and eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P261, P264, P272, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 230°C |
| LD50 (median dose) | LD50 (median dose) >2000 mg/kg (rat, oral) |
| NIOSH | KMZ35680 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for N-(Carbamoylmethyl)Taurine: Not established |
| REL (Recommended) | 0.2 mg/m³ |
| Related compounds | |
| Related compounds |
Taurine Isethionate Homotaurine |