Long before Morpholinium Toluene-4-Sulphonate found its way into laboratory and industrial supply chains, researchers turned to sulphonated organics to solve a range of technical puzzles. Back in the middle of the twentieth century, the push for more reliable ion exchangers steered chemists toward morpholinium salts, already used in other chemical lanes. Laboratories in the Soviet Union and Western Europe began tinkering with sulphonation of toluene derivatives and morpholine derivatives together, noting the potential for improved thermal stability and ionic conductivity. As energy sectors grew hungry for better electrolytes, morpholinium-based compounds moved from bench science toward scaled syntheses. That blend of discovery and application led morpholinium toluene sulphonates to become building blocks in electronics, batteries, and advanced materials.
Morpholinium Toluene-4-Sulphonate falls in a class of organic salts with a restricted but very focused range of applications. Its structure—pairing a morpholinium cation and the toluene-4-sulphonate anion—offers a mix of water solubility and decent thermal stability, qualities that have attracted several R&D streams. Firms producing advanced resins and ionic liquids lean on such compounds for their unique combination of stability and ion transport, a property that shows up in everything from coatings and sensors to lithium battery processes.
Solid at room temperature, this salt usually appears as a fine crystalline powder, white to off-white, depending on purity and production method. With a melting point that often sits near 200°C, Morpholinium Toluene-4-Sulphonate stands up under high-temperature synthesis steps. The substance dissolves easily in water, with partial solubility in some polar organic solvents. Chemically, the morpholinium ring interacts with sulphonate’s anionic charge, locking the salt into a very stable configuration. That structure fends off decomposition in ambient air and resists hydrolysis during handling. The pH of a saturated solution usually slips a bit outside neutral, generally resting slightly acidic, a factor relevant for both storage and end-use formulation.
Manufacturers often supply this salt in high purity grades, listing key specs such as assay (often greater than 98%), water content, and trace metal impurities. Labels cite the CAS number, structure, and batch-specific details, including recommended storage conditions—cool, dry, and in sealed packaging. Containers typically hold 100 grams to several kilograms, depending on whether the buyer works at bench scale or pilot-plant level. SDS sheets highlight the need for gloves, goggles, and local exhaust ventilation whenever transferring the powder or making up solutions. Regulatory identifiers, including REACH registration in Europe and TSCA inventory in the United States, allow end-users to check the compound’s compliance profile before bringing it into inventory.
The most common route takes morpholine and an appropriate toluene-4-sulphonic acid, combining the two under aqueous conditions. This acid-base neutralization finishes quickly, as morpholine is both a good base and a willing participant in salt formation. Careful pH control helps drive the reaction to completion with minimal side products. Filtration and washing remove any excessive toluene sulphonate, followed by drying under vacuum or gentle heat. With a well-practiced hand and standard lab glassware, the synthesis takes only a few hours, making this salt an accessible target for both synthetic chemists and process operators needing reliable intermediates on short timelines.
Morpholinium Toluene-4-Sulphonate resists most mild oxidizers and reducers but will react strongly with Lewis acids or powerful bases. Chemists can swap out the toluene-4-sulphonate anion with other sulphonates through ion exchange, tuning the physical properties for specific uses. Under alkaline conditions, the morpholinium ring can open but only at elevated temperature or prolonged exposure. Electrophilic or nucleophilic attacks on the aromatic ring rarely proceed cleanly, so most modifications steer clear of such pathways. The salt’s ionic nature stabilizes it in aqueous solution, supporting everything from direct electrodeposition experiments to dopant work in polymer matrices.
In the trade, the compound turns up as Morpholinium Tosylate or Morpholinium p-Toluenesulphonate. Some product catalogs call it 4-Methylbenzenesulfonic acid morpholinium salt. Registry numbers and supplier-specific codes round out the labeling, but all point back to the same core structure—a morpholinium cation paired with a tosylate anion.
Direct skin or eye contact leads to irritation, and dust inhalation poses a respiratory risk if handled in poorly ventilated areas. Material Safety Data Sheets recommend gloves, goggles, dust masks, and fume hoods when planning a scale-up procedure. Spill cleanup needs methodical scooping, avoiding water sprays that could spread fine dust. Fire hazards remain low, but steely focus on storage and handling rules protects both the operator and the work environment. All containers require clear labeling, and emergency eyewash stations must stay within immediate reach for anyone working at the receiving dock or batch mixing zone. As with any morpholinium salt, the risk climbs when mixing with strong acids or bases, so storage segregation and clear process documentation carry real stakes.
Morpholinium Toluene-4-Sulphonate traces its most critical uses into electrolyte design for batteries and electrochemical cells. Materials scientists like its stable conductivity and compatibility with both aqueous and organic solvents. Beyond batteries, the salt shows up in antistatic coatings, phase-transfer catalysts, and as a building block in advanced resin chemistry. Some polymer makers add it to tweak ion mobility, chasing better performance in membranes and sensors. Researchers keep probing the boundaries, looking at potential uses in nanocomposites, smart textiles, and thermal stabilizers for specialty materials.
University labs and corporate R&D teams have spent decades exploring morpholinium salts for niche but high-value applications. Reports show steady growth in new electrolyte formulations that use Morpholinium Toluene-4-Sulphonate, especially as grid energy storage and electric vehicle batteries look for longer cycle lives and fewer flammable components. Early work on water-based batteries pointed to this salt as a key candidate, driving up procurement by start-ups and blue-chip chemical producers alike. Patent filings track a wide range of modifications—adding side chains, blending with other ionic liquids, and pairing with novel polymers—expanding what might be possible in next-gen electronics and membrane technologies.
Acute toxicity stays low based on available animal studies, but long-term exposure hasn’t been fully mapped out yet. Chronic effects demand more attention, especially as the compound spreads in high-throughput manufacturing. Tissue sensitivity tests show mild irritation, and environmental fate studies suggest the salt dissolves readily, raising questions around aquatic toxicity in case of large spills. Regulatory bodies haven’t flagged it as a major environmental hazard, but prudent stewardship—collecting wash water, treating effluent, keeping unused stocks secure—remains basic good sense. For lab and plant staff, routine health screening and clear workplace SOPs do more heavy lifting than any single regulatory filing.
As renewable energy and advanced composites move up every government’s wish list, demand for stable, efficient ionic conductors only grows. Morpholinium Toluene-4-Sulphonate holds a place in that pipeline, especially as battery makers reach for safety, robustness, and cost stability. Scientists now blend this salt with nanoparticles, aiming for breakthrough results in conductivity and durability. Regulatory clarity will matter more as tonnage volumes climb, prompting producers to dig into supply chain traceability and green chemistry tweaks. Emerging uses in printed electronics and membrane filtration stretch the boundaries of what was once a dusty laboratory staple. Growth will depend on investment in clean, high-output preparation routes, full-spectrum toxicology, and collaboration across both academic and industrial sites. This salt started life as a technical curiosity, but the march of research points to a wider, and greener, future.
Plenty of people have never heard of morpholinium toluene-4-sulphonate. Truth is, if you ask someone outside of chemistry circles, you’re likely to get a blank stare. Yet, inside research labs and specialty manufacturing plants, this compound pops up in some key roles. My years tangled up in materials science showed me that nearly every substance with a tough name hides real stories behind its formula.
I first came across morpholinium toluene-4-sulphonate at a start-up looking to make better electrochemical sensors. This salt, made from morpholine and toluene sulfonic acid, holds a solid place in the world of ionic liquids and electrochemistry. In these environments, the reliability of a salt—how well it dissolves, how stable it stays—can make or break a project. Researchers value this chemical for helping to tweak conductivity in batteries, fuel cells, and even supercapacitors. Not every salt plays well in high-energy systems, but this one tends to offer predictable behavior, which keeps engineers and scientists coming back to it.
Beyond batteries, it enters the polymer field. Morpholinium salts like this one work as catalysts and stabilizers during chemical reactions. Think adhesives, resins, coatings—areas where a slight edge in consistency or reaction speed equals better products. Factories always want to cut waste and control costs, so when a salt steps in to boost yield or shelf life, it’s got a spot in the lineup. I’ve talked with polymer engineers who have switched additives simply because of issues with residue or slow curing. Morpholinium salts, thanks to their ionic structure, often help sidestep those headaches.
Every new material sparks questions about safety, at least from anyone with experience in manufacturing. Morpholinium toluene-4-sulphonate carries lower volatility compared to many traditional solvents and additives. This means less mess in the air and fewer headaches (literally) for workers. I learned over the years that facilities that prioritize worker safety and environmental health usually face fewer fines and enjoy stronger loyalty from employees. Regulations around chemicals tighten year after year, so every edge counts.
Still, chemical disposal stays complicated. Even a “safer” compound needs proper handling at the end of its useful life. Environmental scientists pay close attention to what washes out of factories and labs. For a compound like morpholinium toluene-4-sulphonate, the path to truly green chemistry calls for investment in reclamation and recycling. It’s something I saw growing as a priority in both large corporations and nimble start-ups—mainly because waste disposal now directly impacts profits and brand perception.
Industries could push harder for greener synthesis methods for this and similar compounds. Academic labs sometimes crack new ways to produce these salts without harsh solvents or expensive purification. If large-scale manufacturers take cues from those processes, costs drop, and environmental impact shrinks. From my experience, the companies quick to implement small process tweaks usually outlast the competition when market prices shift or new rules hit.
Morpholinium toluene-4-sulphonate might never become a household name, but for people solving tomorrow’s technology challenges, it earns its spot in the toolkit. Focusing on responsible sourcing and thoughtful disposal can make it a better fit, both for industry needs and for the planet’s well-being.
Morpholinium Toluene-4-Sulphonate shows up regularly in labs or chemical plants thanks to its unique properties. Safety always plays a big part in handling chemicals, and storing them correctly deserves special attention. Mishandling brings risk, not just to workers but to the quality of the final output and the surrounding environment.
If you’ve ever tried stacking your home supplies in a humid basement, you probably remember how things get sticky and clump together. Morpholinium Toluene-4-Sulphonate faces a similar problem. Moisture triggers changes in the compound, which could lead to degradation or clumping. For this reason, folks who work with it use air-tight containers—preferably made from high-density polyethylene or glass—to seal out humidity and airborne contaminants. These containers should stay off the floor to cut down on temperature swings and leaks.
Nobody likes returning to a storeroom and finding that sunlight has faded or changed the products on a shelf. Direct sunlight can slowly change the makeup of certain chemicals. For this compound, a cool, dry, shaded area works best. Shelving should sit far from windows and in rooms with consistent temperature, ideally below 30°C. Chemical storage rooms with air conditioning or dedicated ventilation help prevent the build-up of harmful vapors and keep temperature steady.
Chemical compatibility matters just like sorting recycling at home. Combining compounds that react violently can lead to spills and accidents. Morpholinium Toluene-4-Sulphonate reacts with strong oxidizers or certain acids, which could cause fires or toxic smoke. Always separate it from incompatible substances. Color-coded shelving, clear signage, and digital inventory tools can help staff avoid mistakes during storage or retrieval.
Anyone who stores or handles this material needs proper personal protective equipment: gloves, eye protection, and lab coats. Even quick exposure can cause irritation or worse if it lands on skin or in eyes. Accidents do happen, so ready access to an eyewash station, spill kits with absorbent materials, and clear clean-up protocols should be part of every storage site.
Local and international regulations set out strict rules for the storage of chemical substances, including Morpholinium Toluene-4-Sulphonate. OSHA, REACH, and local environmental health agencies recommend regular safety checks, clear hazard labeling, and up-to-date records of chemicals onsite. These aren’t just bureaucratic hurdles—they reduce risk for everyone on the property.
A cluttered, neglected storage room invites problems. Routine inspections flag expired or compromised stock, containers with missing labels, and blocked exits. Investing in proper racking, clear marking, and personnel training makes a safer workplace for all. Clear emergency response plans also pay off the day something does go wrong.
Temperature- and humidity-controlled cabinets offer peace of mind for those storing heat or moisture-sensitive materials. Digital thermometers and hygrometers provide constant feedback. Training workers on chemical hazards and safe handling techniques, plus encouraging a culture where people report storage issues, limits the chance of accidents. Simple changes like locking storerooms and using checklists take the guesswork out of safety.
Morpholinium toluene-4-sulphonate turns up in a handful of specialty chemical processes. Anyone who's worked in a chemistry lab has probably seen compounds with complicated names like this, but complexity in name doesn't always equal danger. People want to know, “Am I safe working around this stuff?” That's not an unreasonable question, especially for those who handle chemicals every day or for people living near factories where these materials come into play.
Based on available data and regulatory sources, morpholinium toluene-4-sulphonate isn’t among the top-tier hazardous chemicals like cyanide or benzene. Its main risk points involve direct contact, inhalation of dust, or accidental ingestion. As with most organic salts, it can cause irritation on the skin, in the eyes, and to the respiratory system. Long-term toxicity studies on this compound don’t turn up much in the research databases, probably because it hasn’t flooded the global market like some other chemicals have. That absence doesn’t mean a green light for careless handling. It just tells us that the red flags haven’t shot up—yet.
With any industrial or specialty chemical, context matters. Lab safety protocols treat every unfamiliar powder or crystal with respect for a reason. Some compounds only reveal problems after repeated exposure—think about solvents that looked safe until workers got sick years later. Morpholinium toluene-4-sulphonate hasn’t been linked to cancer or reproductive harm according to safety sheets and the Globally Harmonized System (GHS). But the irritation risks shouldn't be downplayed, and allergic reactions always lurk as a possibility for some individuals.
I’ve seen first-hand how some chemicals with boring safety labels still trip up young chemists just out of school. Rushing through cleanup, or skipping gloves “just this once” turns a routine day sour. If this compound comes as a powder, it can linger in the air or cover work surfaces. Even small exposures, repeated day after day, have a way of building up health complaints. Nosebleeds, itchy eyes, unexplained coughs—been there, seen it plenty. People rarely get seriously hurt if they follow the guidelines: gloves, goggles, and decent ventilation.
Some chemicals slip under the radar because they’re not mass-produced, or because they’re used behind closed doors in industry. Morpholinium toluene-4-sulphonate doesn't attract bans from OSHA, EPA, or Europe’s REACH authorities as of now, but manufacturers still post hazard warnings because the basic toxicology supports them. The right way to treat a substance like this involves a mindset that admits what we don’t know—especially around new uses or higher volumes.
If companies want to keep their workers safe, transparency and routine safety training make the biggest difference. Material Safety Data Sheets don’t belong in a dusty filing cabinet; they should be easy to grab, read, and talk about. For researchers, responsible disposal and spill plans must stay more than a formality. For families living near chemical plants, local governments should keep tabs on emissions and make it easier to report lingering odors or dust problems. People sometimes need to push facility managers for clear answers, and it helps to know exactly what’s being stored or processed down the street.
Giving morpholinium toluene-4-sulphonate the respect it deserves doesn’t mean spreading panic. It means treating every chemical’s risks seriously, even if the science hasn’t filled in every blank yet. Gloves, goggles, and regular training beat luck every time.
Morpholinium Toluene-4-Sulphonate rarely sits on the household shelf. Research labs and some industrial settings use it for specific reactions or processes, and anyone coming into contact with it deserves straight talk on safety. Exposure risks aren’t just small talk—studies point out respiratory and skin irritation, and accidental swallowing brings real trouble. To get safety right, it’s not enough to skim a data sheet; personal experiences and the lessons learned from missteps matter most.
Some years back, I worked alongside a chemist who handled compounds like Morpholinium Toluene-4-Sulphonate daily. She made safety goggles and a snug pair of chemical-resistant gloves part of her work routine. Cotton or latex offered little defense, so nitrile or similar lab-grade gloves worked best. That focus on proper gear meant skin stayed protected and eye injuries stayed theoretical. Good ventilation—think hoods and extraction fans—doesn’t just move air; it reduces inhalation risk. Relying on open windows or hoping air will clear on its own never cut it, especially for compounds that aren’t household names.
People sometimes treat chemical labels as a box-ticking exercise, but understanding hazard codes saves more than time. The Health and Safety Executive (HSE) and the Occupational Safety and Health Administration (OSHA) both list Morpholinium Toluene-4-Sulphonate for its corrosive qualities. Handling it carelessly, with bare hands or in a stuffy room, pretty much invites trouble.
Standard advice gives a nod to eyewash stations and showers, but who checks if water pressure’s up to scratch? An eyewash station gathering dust can’t help anyone. Auditing lab safety gear and running actual drills should top the list. Too many hospital visits happen because people thought a quick splash or fume exposure wouldn’t matter. I’ve seen colleagues wash off unknown splashes with soap and water, and that simple step has paid off every time trouble was brewing.
Over time, one thing stands out: chemical handling isn’t just about protecting yourself. Spills spread fast. Morpholinium Toluene-4-Sulphonate doesn’t evaporate quietly; it leaves residues and those residues travel on clothing and shoes. One spill on tile can find its way to public spaces or someone’s handrail. That risk grows in busy labs where traffic brings in cross-contamination.
Plenty of headlines blame “human error” but don’t look close at what causes slips. Most mistakes connect back to rushed work, unclear protocols, or poor communication. Repetition in training helps, but mock scenarios stick even better. Spill kits with clear instructions, and real-time walk-throughs, let people react fast when things go wrong.
Clear labeling, good lighting, and uncluttered work surfaces mean fewer accidents. In one workplace, we set up a buddy system where people checked each other’s PPE before opening containers. It felt awkward for a week, then became second nature. The point spread to other hazards too—anything that cut down accident rates proved its worth with fewer incidents and less downtime.
Safe handling of Morpholinium Toluene-4-Sulphonate isn’t about memorizing rules; it’s about investing in a culture where caution wins out over convenience. Watching out for each other and learning from every near-miss does more than any sign on the wall. More investment in routine training, transparent reporting of near misses, and sharing stories within teams will drive home the risks and the simple actions that keep everyone safe.
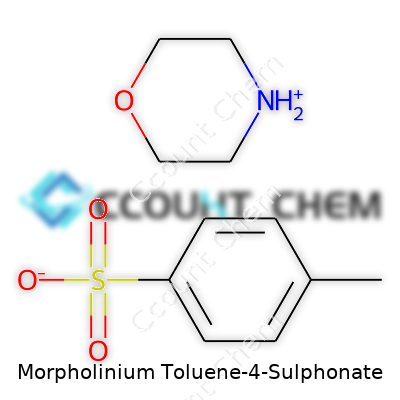
In chemistry, a mouthful like "Morpholinium Toluene-4-Sulphonate" is more than just a complex label. This compound brings together two distinctive chemical groups: morpholinium and toluene-4-sulphonate. Breaking down what these pieces represent can help demystify its role and usefulness.
The morpholinium cation comes from morpholine, a six-membered ring with four carbons, one oxygen, and one nitrogen. If you've worked in a lab, you’ve probably caught its signature odor or used it as a solvent. Add an extra hydrogen to that nitrogen, and now you have morpholinium, carrying a positive charge.
On the other side sits the toluene-4-sulphonate anion. The toluene ring holds a methyl group at the first position and a sulphonate group at the fourth. With the aromatic ring, the compound lands in a sweet spot for stability and reactivity, which helps make the salts of these molecules reliable in chemical processes.
Put these together and you get the full structure: a morpholinium cation paired with a toluene-4-sulphonate anion.
The combined molecular formula sums up the elements involved: for morpholinium, C4H10NO+, and for toluene-4-sulphonate, C7H7SO3-. When paired as a salt (one positive, one negative for charge balance), the full formula becomes C11H17NO4S. Some of us who have spent time in analytical chem labs know the frustrations of balancing charges and counting atoms, but this one’s relatively straightforward.
Digging into the structure might sound academic, but for real-world applications, it’s practical knowledge. The morpholinium cation is known to play nicely with a wide variety of anions, which opens doors for custom-made ionic liquids. Ionic liquids are showing up in electrochemistry and green chemistry, from extraction and synthesis to heat transfer. That speaks to both the versatility and the significance of compounds like morpholinium toluene-4-sulphonate.
Chemists and engineers working in battery research or catalysis often look for new “designer” salts to push the envelope on safety, efficiency, and environmental impact. This particular pairing holds up against moisture, resists degradation, and gets along well with other compounds, making it a strong candidate for those sorts of applications.
Data from safety sheets and journals show that morpholinium-based salts often have lower toxicity compared to other organic solvents, which is important from a workplace safety perspective. Plus, they don’t break down as easily when heated or mixed with water. Handling rules still matter—these salts are not risk-free. Handling with gloves and proper ventilation is standard best practice.
Access to precise chemical information is crucial for researchers and industry. Gaps in data can slow innovation and lead to costly mistakes. Manufacturers and laboratories can help by providing high-purity samples and transparent sourcing.
One significant issue lies in clarity and standardization of information. Open access chemical databases, improved labeling, and direct communication between suppliers and end-users can improve reliability. Labs that share experiences and publish negative results alongside successes help the broader community understand where certain compounds shine—and where they don’t.
Young chemists and seasoned professionals both depend on accurate, honest knowledge. Staying curious and willing to share practical lessons not only pushes research forward—it improves safety and trust across the board.
| Names | |
| Preferred IUPAC name | 4-methylbenzenesulfonic acid; morpholine |
| Other names |
4-Toluenesulfonic acid morpholine salt Morpholine p-toluenesulfonate Morpholine tosylate Morpholinium p-toluenesulfonate Morpholinium 4-methylbenzenesulfonate |
| Pronunciation | /ˌmɔːrˈfɒlɪniəm təˈluːiːn fɔːˈsʌl.fə.neɪt/ |
| Identifiers | |
| CAS Number | 3162-58-1 |
| 3D model (JSmol) | `3Dmol.js,GLmol,load=pubchem:24894242` |
| Beilstein Reference | 1125056 |
| ChEBI | CHEBI:87673 |
| ChEMBL | CHEMBL514984 |
| ChemSpider | 22213568 |
| DrugBank | DB13520 |
| ECHA InfoCard | 100.102.717 |
| EC Number | 262-087-1 |
| Gmelin Reference | 114139 |
| KEGG | C18607 |
| MeSH | D019273 |
| PubChem CID | 24459637 |
| RTECS number | VP2275000 |
| UNII | 4135892LTG |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DJ3JK2A9V2 |
| Properties | |
| Chemical formula | C4H10NO•C7H8O3S |
| Molar mass | 261.32 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.28 g/cm3 |
| Solubility in water | Soluble in water |
| log P | −2.2 |
| Acidity (pKa) | 4.8 |
| Basicity (pKb) | 6.0 |
| Magnetic susceptibility (χ) | -73.0e-6 cm³/mol |
| Refractive index (nD) | 1.546 |
| Viscosity | Viscosity: 1500 cP |
| Dipole moment | 6.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.74 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −1239.1 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4155.7 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H318, H319, H335 |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 107°C |
| Lethal dose or concentration | LD50 (oral, rat): 1800 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1800 mg/kg (oral, rat) |
| NIOSH | WJ6300000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not established |
| Related compounds | |
| Related compounds |
Morpholine Toluene-4-sulfonic acid Morpholinium chloride Pyridinium toluene-4-sulfonate Triethylammonium toluene-4-sulfonate |