Methylsulfonic acid didn’t just appear out of nowhere—early chemists first isolated it in the 19th century when industrial processes began experimenting with organosulfur compounds. By the time the 20th century rolled around, its usefulness in electroplating and as a catalyst stood out. Its adoption in synthetic chemistry followed, with pharmaceutical and electronics industries pushing for a steady supply. Over the decades, improvements in purification and synthesis have made it more accessible and safer for both lab-scale and industrial operators.
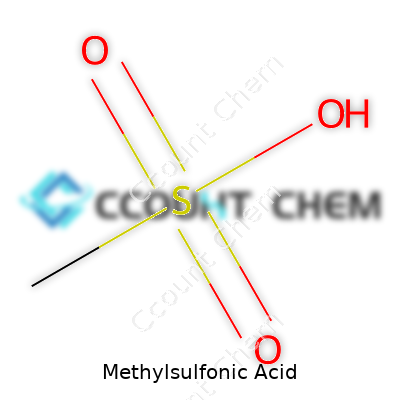
Methylsulfonic acid, also called methanesulfonic acid, packs a punch in both strength and versatility. Its formula—CH3SO3H—is straight to the point, showing a simple backbone with a strong acidic function. It brings the benefits of mineral acids without the volatility of options like sulfuric or hydrochloric acid. Companies sell it in both anhydrous and aqueous formats with purity levels often exceeding 99%, as demanded by electronics manufacturing and chemical synthesis experts.
You’ll spot this compound as a colorless, odorless liquid under normal conditions, though solid flakes form in colder environments. It boils at 167°C under pressure and sits comfortably in water, ethanol, or ether. Its strength as an acid puts it on par with hydrochloric acid, but unlike some other strong acids, it doesn’t throw off pungent fumes. It resists oxidation in air and keeps a stable profile in most settings, which helps manufacturers avoid some nasty surprises. Given its strong acidity, it strips away inorganic residues without the aggressive side effects of mineral acids.
On a spec sheet, methylsulfonic acid finds itself labeled with product purity, water content, color, and non-volatile residue. Reputable suppliers disclose concentrations of trace metals—essential information for anyone in electronics or pharmaceutical manufacturing where ionic contamination spells trouble. Labels feature standard hazard pictograms and regulatory information according to GHS and REACH, warning users about the need for protective gear and ventilation but not overstating the risk to those familiar with handling strong acids.
Industrial routes keep things efficient: methylsulfonic acid forms when methanethiol, itself a byproduct from natural gas, reacts with oxidizers like hydrogen peroxide or chlorine. Lab-scale preparation relies on oxidizing dimethyldisulfide or dimethylsulfide. Equipment demands corrosion-resistant alloys and fume extraction, but the chemistry itself isn’t so harsh that it strains modern plant design. Any operator with experience managing acid manufacturing will spot the familiar safety patterns and process controls at work.
This acid doesn’t rest on its own laurels. In the lab, it reacts cleanly with alcohols to form methylsulfonate esters, which pop up as intermediates in pharmaceuticals and agrochemicals. It activates carbonyl compounds for nucleophilic attack and can help introduce sulfonic acid groups into organic rings. Chemists favor its gentleness towards sensitive molecules, making it a go-to catalyst for esterifications or etherifications where stronger acids scorch the target. With the right tweaking, its chemical backbone lets researchers synthesize a range of useful salts and derivatives.
Known by many names, the substance answers to methanesulfonic acid, methylsulphonic acid, and MSM acid in the pharmaceutical trade. CAS number 75-75-2 shows up everywhere from lab catalogs to regulatory papers. Brand names may dress it up, but the core compound stays the same, which lets buyers focus on quality and purity instead of navigating unnecessary confusion.
In my own experience working with acids, methylsulfonic acid stands out for being less volatile than popular mineral acids but still demands caution. Direct contact causes skin and eye burns, so gloves and goggles are non-negotiable. Ventilation keeps inhalation risks low, but splashes create painful incidents if procedures slip. SDS guidance stresses careful storage, away from bases and oxidizers. Labs and plants maintain spill response kits and washing stations; ignoring them leads to stories best avoided. Proper labeling and regular training help prevent the kind of mistakes that damage equipment or, worse, people.
Methylsulfonic acid earns its reputation as a multi-tool. It sits on the workbench in electroplating shops, ensuring uniform metal coatings in semiconductor fab rooms and jewelry workshops. Chemists reach for it as an acid catalyst, especially in pharma and specialty coatings, because it avoids charring molecules sensitive to higher temperatures or oxidizing agents. Water treatment plants even use it to tweak pH or solubilize metals. In my time supporting electronics clients, the compound’s predictability and cleanliness sealed the deal—no surprise shifts in pH or side-reactions that drag down product quality.
Labs keep finding new ways to employ methylsulfonic acid. As green chemistry pushes industries to phase out more aggressive or hazardous acids, researchers look at its breakdown profile and residue control with fresh interest. Teams test milder process conditions for polymerization, pharmaceutical synthesis, or energy storage systems. Its strong acid properties, mixed with low toxicity to the environment, encourage innovation in recyclable batteries and safer pharmaceutical intermediates. Universities and commercial labs both keep pushing for catalytic cycles that lower waste and energy use, and methylsulfonic acid often features in prototypes and patents.
On the toxicology front, this is one area where long-term studies help. Acute exposure, like a splash in the eye, brings the expected burns, but repeated low-level exposure doesn’t appear to accumulate in tissues, owing to its rapid excretion as sulfate. Animal research has flagged mild pulmonary and gastrointestinal effects after chronic exposure, but its lack of persistent organic pollutants makes it a favorite over halogenated acids or those prone to bioaccumulation. Regulatory reviews keep a close eye on new evidence, but so far, methylsulfonic acid avoids stigma compared to many older industrial acids.
Looking farther out, I see growth ahead as more industries hunt for efficient and cleaner acids to streamline production. The electronic materials sector keeps expanding, and methylsulfonic acid’s track record in offering defect-free soldering and plating stirs interest globally. Pharma companies want catalysts that don’t introduce heavy metals or problematic anions. New energy projects—think fuel cells and advanced battery chemistries—are testing its compatibility for stable and safe electrolytes. As chemical engineers and regulatory agencies tighten standards for workplace exposure and discharge, methylsulfonic acid stands to secure its place as a reliable option where strength, selectivity, and environmental responsibility all matter.
Methylsulfonic acid stands out on the chemical shelf because people reach for it when they need a strong acid that won’t corrode stainless steel or react in unexpected ways. In factories and labs, it cleans up messes that weaker acids can’t touch. Plumbers and metalworkers trust it for descaling boilers and treating metals since it cuts through buildup without damaging the underlying surfaces.
In electronics, everything starts with precision, and methylsulfonic acid delivers that by helping keep circuit boards free from contaminants. Manufacturers lean on it to prepare metal surfaces before plating or soldering—a clean start means fewer failures later. Its ability to dissolve minerals and oxides gives tech hardware a longer life and fewer shorts, and that keeps devices running longer. It’s pretty impressive that something so straightforward saves companies thousands by reducing repairs and waste.
Pharmaceutical labs can’t take risks with impurities. Methylsulfonic acid works as a catalyst in drug manufacturing since it promotes reactions but won’t leave behind harmful by-products. It also helps in cleaning reactors between batches, which prevents cross-contamination. Drugs need to be safe and pure, and the choice of cleaning chemicals can make or break a production line. According to industry data, strong acids like this one help companies meet safety standards and avoid expensive recalls. I’ve seen the difference that reliable cleaning acids make in labs—people feel more confident running trials and scaling up production.
The energy sector takes methylsulfonic acid seriously when making electrolytes for batteries, especially in high-end applications. Unlike sulfuric acid, it doesn’t produce as much gas or heat, so battery manufacturers can make safer and more durable products. As the world shifts toward electric vehicles and solar storage, safer battery production matters. Using this acid means workers have fewer accidents, and the environment gets a break from unnecessary leaks or spills.
Methylsulfonic acid has real punch, and that’s why safety checks make a difference. A splash can burn skin, and the fumes can irritate lungs. Workers wear gloves, goggles, and sometimes even full-face shields. Companies keep neutralizers and clean water nearby. Regular training helps prevent accidents. People make mistakes, sure, but strong safety culture turns dangerous jobs into routine days at work. Strong acids only cause harm when people skip steps or don’t respect what the materials can do.
Disposing of chemicals from factories and labs isn’t simple. Methylsulfonic acid breaks down more easily than many other strong acids, which lowers the risk to waterways if it’s handled properly. Companies that recycle or neutralize waste help keep harm out of rivers and soil. Some research even points toward greener alternatives in the future, but nothing matches its combination of strength and gentleness toward metals just yet.
People keep searching for new ways to make chemical work safer for both workers and the world beyond the facility gates. Methylsulfonic acid stands as proof that strong tools can fit into careful hands. If the industry continues investing in safety and cleaner technologies, the benefits reach everyone who plugs in a device, depends on clean medicine, or commutes in an electric car.
Methylsulfonic acid pops up in labs and factories more often than many people realize. It’s strong, clear, and heavy in the hand, and its acidity outpaces even some legendary industrial acids. Those details catch chemists’ attention right away, but storage and handling often get less focus than they should. People working with chemicals day in and day out sometimes relax their guard, and that’s where problems start.
Almost every workplace has stories where acid storage made or broke safety records. Years ago, I watched a team stash containers of methylsulfonic acid near water lines and untreated steel shelves. The labels said “Corrosive” but those words faded into the background after months on the shelf. One hot, humid afternoon, the plastic drum showed bulging seams and started to drip. Acid vapor, invisible but biting, stung at the eyes and nose. Someone had to don protective gear and neutralize the spill before the building’s integrity took a hit. All that hassle grew out of ignoring some straightforward storage rules.
Methylsulfonic acid chews through the wrong package. High-density polyethylene, polytetrafluoroethylene (PTFE), and glass all stand up to it. Never trust mild steel, low-grade plastics, or any metal that isn’t specifically rated to handle acids. I’ve seen metal drums leach rust and holes in months when someone used a container from the wrong delivery, thinking it wouldn’t matter for a few weeks. That quick decision ended with a surprise leak in a warehouse corner.
This acid craves cool, dry, and well-ventilated spots. Keep it clear of moisture. Water pulls fumes out fast and ramps up the risk when lids come off. Storage temperature shouldn’t swing much—stable, moderate climate slows down container fatigue. Always place acids below shoulder level in chemical storage cabinets built for corrosives, with secondary containment trays underneath. Even the best lab hand can fumble, so the best setups plan for splashes or leaks before they happen.
Anyone lifting or pouring methylsulfonic acid grabs gloves rated for chemical resistance, full-face shields, and acid-resistant aprons. Regular latex or nitrile gloves give out, especially after long contact. I once visited a plant where workers used cheap gloves and learned the hard way that even a few minutes of exposure leads to redness and burns. Goggles alone don’t offer enough defense; vapor creeps in from the sides. Full-face shields and proper ventilation avoid those trips to the emergency room.
Corrosive spills can ratchet up from small to severe if there’s panic or confusion. It pays off to have acid neutralizer, spill kits, and eye wash stations close by. Not just in a main lab either—storage areas need first response tools within a few steps. After seeing how fast corrosion eats through floors and walls, I always recommend regular inspection of storage spaces for acid residue, weakened shelves, and exhaust systems that haven’t run in weeks.
There’s equipment and training, but the most important factor comes down to attention. Routine walk-throughs, checking drum seals, updating labels, and talking about real accidents sharpen that focus. People working near methylsulfonic acid carry the responsibility to bring up problems when they spot them. No shortcut replaces steady vigilance. Safe daily habits will always outrun fancy equipment or rules written in a binder.
Methylsulfonic acid, or methanesulfonic acid as some call it, shows up in many industrial cleaning products and processes. This clear liquid carries a tough reputation for dissolving tough stains, helping with metal finishing, and acting as a catalyst for various chemical reactions. On paper, everything about this substance suggests efficiency. The concern often shows up after digging into the risks for the people working with it and the impact if it gets into local waterways or soil.
Direct skin contact with methylsulfonic acid burns. Its vapors sting eyes and can damage lungs if someone inhales it without protection. In factories, workers must wear gloves and face shields, and use fume hoods to stay safe. This level of caution exists for good reason. Severe exposure does not lead just to discomfort; in some cases, it means permanent scarring. I spent time talking with a technician from a plating shop who described the itch and redness that followed the tiniest splash under his glove. Since then, his shift always starts after double-checking his gear.
Nobody wants this stuff getting into a cut, and nobody ignores a spill. The acid reacts with many metals, releasing hydrogen, which can turn a workplace into a dangerous zone quickly. Regular safety training stands between routine productivity and an accident that could ruin lives. Still, strong rules and protective equipment work. Accidents happen less often in facilities that drill these habits into everyone on the floor.
Acid spills in the lab are easy to contain. Out in the environment, these spills can turn streams and ponds acidic fast. Methylsulfonic acid breaks down in water, making it biodegradable in small amounts. That’s good news, but it doesn’t erase what happens to fish or amphibians living in water that sees a sudden drop in pH. Some die, eggs fail to hatch, and plants in the water turn yellow and rot.
Cities that have chemical plants often check wastewater before it leaves the facility. Local stories about mysterious fish kills sometimes link back to a leaky pipe or a storage tank that overflowed. Nobody ever finds out after a day—it usually takes weeks for a clear answer to emerge. Families who use downstream water would prefer to never see this happen in the first place.
Safe chemical handling comes down to training, equipment upkeep, and believing that nothing is too unlikely to prepare for. Companies choose acid alternatives for cleaning and finishing where possible—citric acid or phosphate options step in where performance allows. If methylsulfonic acid stays necessary, workers use neutralizing stations and handle neutralized waste like it’s still dangerous.
Oversight from local agencies makes a difference. Firms that skip quarterly safety drills or hide small leaks face fines that hurt the bottom line. Technology helps, too. Sensors and remote alarms catch leaks before anyone puts on a raincoat and heads into the plant. At the city scale, stricter discharge limits and real-time monitoring keep contamination from becoming news in the first place.
Methylsulfonic acid makes industry run smoother in the right setting, but treating it like just another cleaner piles on health risks and environmental threats. Run the line with respect for the risks, back it up with safe practice, and both people and nearby creeks come out better for it.
Methylsulfonic acid’s chemical formula is CH4O3S. Its molecular weight clocks in at about 96.10 grams per mole. Those details matter more than many realize. I’ve worked alongside lab techs who always check formulas and weights, not because they want to sound smart, but because every number counts. Slip up, and you risk sending an experiment off the rails or ruining a crucial reaction that costs the company big time.
In industry, labs don’t run on guesswork. Say you’re using methylsulfonic acid as a catalyst, which happens in some organic syntheses. The difference between getting a reaction to work safely or creating a big mess sometimes comes down to a half-gram slip in weight calculation. Formula errors can expose workers to unexpected hazards or lead to poor product yields. I’ve witnessed entire teams halt production to re-examine these details after a formula was copied incorrectly. No one enjoys that, especially when deadlines loom.
Chemicals like methylsulfonic acid aren’t just lab curiosities. They show up in pharmaceuticals, electroplating shops, and sometimes even in specialty cleaning agents. In these settings, the right formula isn’t just a number on a bottle—it’s about health and safety. I recall talking with a wastewater technician who handled mishaps because a supplier mislabeled drum contents. Mix up CH4O3S with something else, and downstream systems can corrode before anyone catches it.
Regulatory agencies take this seriously for good reason. For example, OSHA and the EPA outline safety requirements based around a chemical’s identity. Companies that use methylsulfonic acid responsibly keep updated Safety Data Sheets (SDS) close at hand and audit those documents to reflect the correct formula and molecular weight. These aren’t just paperwork exercises. They help protect lives, and they guard the environment from preventable accidents.
Precision starts with education. Chemical handlers need training that gets into the nuts and bolts of formulas and weights. It’s not enough to hand out a binder or expect people to memorize a table. Practical refreshers, hands-on demonstrations, and real-world scenarios keep the numbers meaningful. I’ve found that double-checking a formula before mixing two reagents becomes second nature after enough practice—especially if a teacher or foreman shares stories of what goes wrong.
Technology helps minimize errors, too. Digital reference systems, bar-code tracking, and error-checking software catch typos or transposed numbers. Supervisors who trust these systems still encourage their teams to think critically and speak up if something on a label doesn’t seem right.
In the end, accuracy with formulas—and respect for molecular weight—grows trust with co-workers, supervisors, and customers. As someone who’s seen projects grind to a halt over a wrong number, I appreciate those who double-check the basics. Safe and effective work starts with getting the small details—like methylsulfonic acid’s CH4O3S formula and 96.10 g/mol weight—right every time.
Working in labs and chemical plants, I've watched how carelessness turns routine disposal into disaster. Methylsulfonic acid, or methanesulfonic acid, brings more bite than you’d expect from something often used to clean and process metals. Splash it around or dump it down the drain, and you’re risking burns, fumes, corroded pipes, and unwanted reactions that could land someone in an emergency room. Accidents tend to happen when people overlook basics — the “it’s-just-another-acid” attitude creates bigger problems down the line.
Disposal rules for acids like methylsulfonic acid show up in every country’s environmental code. The US Environmental Protection Agency (EPA) flags strong acids under hazardous waste rules. In Europe, the REACH regulation keeps similar tight controls. If a facility ignores these, fines and shutdowns follow — not to mention the damage to water systems or soil and the illnesses in local communities. Dumping acid without treatment finds its way to drinking water or fish habitats before long.
During my time in industrial waste management, I learned that steps start long before you reach for the drain. Staff gear up with acid-resistant gloves, splash goggles, and proper shoes. Containers used for collection don’t react with acid, so high-grade polyethylene or glass works best. Labels go right on the container, describing content and concentration. Check storage areas for good ventilation — no one wants fumes building up.
Getting methylsulfonic acid safe to handle usually calls for careful neutralization. Lab techs and plant workers use sodium bicarbonate, lime, or similar bases. Add the base slowly, stir gently, and keep a close eye because heat and bubbling follow. I’ve watched new workers dump in too much base at once — the violent fizz can spill acid, create toxic vapors, or even break containers. Take it slow, measure pH often, and stop once the liquid sits between pH 6 and 8.
Local environmental rules set limits on what goes down the drain, even after neutralization. Some cities service treatment plants ready for moderate amounts of sulfate-rich water. Others ban it altogether. Never guess — always check with the facility’s environmental, health, and safety (EHS) officer or city water agency. Skipping this step could wreck pipes or harm water treatment bacteria.
In bigger operations, disposal companies collect and haul away neutralized waste for processing at specialized sites. Workers package waste in approved drums. Labels cover contents and hazard info. Pickup gets scheduled with firms licensed to handle corrosive liquids. Trained drivers deliver waste to facilities that treat, recycle, or safely destroy it.
Combining methylsulfonic acid with other chemicals spells trouble. I remember a case where someone mixed it with bleach waste, causing toxic gases to fill the room. Waste streams stay separate to prevent violent reactions and unexpected release of fumes. Double-check every drum, every time, and use secondary containment in case spills happen.
Safe handling starts with training. New workers learn acid dangers and review real-world mistakes. Managers reinforce a safety culture. Signs, emergency showers, and first-aid kits stay stocked and checked. I’ve seen the shift when an organization holds regular drills — people move faster and know what to do if something goes wrong.
Companies keep up with changing laws and new disposal technology. Waste reports get filed and reviewed. If something spills or leaks, staff document it and tweak the plan. Over time, experience and vigilance keep accidents from turning into disasters. Safe disposal isn’t just about following rules — it’s about protecting people and the land we depend on.
| Names | |
| Preferred IUPAC name | Methanesulfonic acid |
| Other names |
Methanesulfonic acid MSA Methanesulphonic acid |
| Pronunciation | /ˌmɛθ.ɪl.sʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 67-56-1 |
| 3D model (JSmol) | `MSMILES("CS(=O)(=O)O")` |
| Beilstein Reference | 1209221 |
| ChEBI | CHEBI:40958 |
| ChEMBL | CHEMBL1406 |
| ChemSpider | 6197 |
| DrugBank | DB13226 |
| ECHA InfoCard | 100.019.133 |
| EC Number | 01-2119537431-46-XXXX |
| Gmelin Reference | 8498 |
| KEGG | C00796 |
| MeSH | D008770 |
| PubChem CID | 639 |
| RTECS number | PV6210000 |
| UNII | T38O14D3GB |
| UN number | UN3265 |
| Properties | |
| Chemical formula | CH4O3S |
| Molar mass | 96.10 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.48 g/cm³ |
| Solubility in water | Miscible |
| log P | -2.0 |
| Vapor pressure | 0.46 hPa (20 °C) |
| Acidity (pKa) | -1.9 |
| Basicity (pKb) | -2.6 |
| Magnetic susceptibility (χ) | -34.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.431 |
| Viscosity | 8.4 mPa·s (25 °C) |
| Dipole moment | 2.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 86.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -567.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -747 kJ/mol |
| Pharmacology | |
| ATC code | D08AX01 |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed, inhaled, or absorbed through skin, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS05 |
| Pictograms | GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P405, P501 |
| NFPA 704 (fire diamond) | 3-0-1-Ac |
| Flash point | Flash point: 185°C |
| Autoignition temperature | 320 °C |
| Lethal dose or concentration | LD50 Oral Rat 1600 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2,500 mg/kg |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 150 mg/m3 |
| Related compounds | |
| Related compounds |
Methanesulfonate Methanesulfonyl chloride Methyl sulfate |