Methyl Toluene-4-Sulphonate has traveled a long way from its origins in the labs of the early 20th century. Back then, the chemical industry was forging ahead, fueled by demand for synthetic intermediates to support the expanding pharmaceutical and dye industries. Scientists studied toluene compounds closely, keen to tweak molecular structures and discover new applications. Methyl Toluene-4-Sulphonate stood out for its reactivity and versatility, earning a place as a valuable building block. The push to improve industrial scale synthesis and purification methods in the 1940s and 1950s shaped the compound’s commercial future, and by the 1970s, its role in fine chemical production grew firmly established.
Methyl Toluene-4-Sulphonate joins the group of sulfonate esters valued for their combination of solubility and reactivity. Manufacturers turn to it for its effectiveness as an intermediate, choosing it when they need to transfer methyl groups or create protected forms of toluene compounds. Its straightforward, well-documented reactivity gives chemists reliability in multi-step synthesis. This reliability often becomes the deciding factor in process chemistry, where unpredictable outcomes cost time and money. So, beyond paper specs, its reputation comes from consistent results, year after year, and that carries weight with every batch produced.
Most will first notice the solid, crystalline form and faint aromatic scent. Methyl Toluene-4-Sulphonate often appears as a colorless to pale yellow powder. Its melting point floats around 70°C to 73°C depending on purity and batch, and it dissolves well in common organic solvents like acetone, ethyl acetate, and dichloromethane but resists water. In terms of chemical behavior, it acts as a good electrophile, especially when interacting with nucleophiles in substitution reactions. The molecular structure—a methyl group at the 4-position of a toluene ring, sulfonated—creates unique performance in key steps across organic syntheses. Consistency in melting point or reactivity flags high product quality, while outliers point to impurities that spark headaches for chemists chasing tight process specifications.
Suppliers mark drums and containers with batch-specific information, so buyers know exactly what arrives in their lab or plant. Typical technical sheets outline purity—usually at or above 98%—along with melting point, residual moisture content, and titration numbers pointing to sulfonate concentration. Chemical safety labels follow global GHS (Globally Harmonized System) guidelines, showing pictograms for irritation or toxicity. Buyers scrutinize these numbers, searching not for generic specs but trustworthy batches that behave the same way every time, since process flare-ups come from small, often overlooked inconsistencies. Accuracy in labeling helps prevent misplaced confidence, a problem anyone managing hazardous substances will recognize.
Crafting Methyl Toluene-4-Sulphonate involves reacting p-toluenesulfonic acid with methanol under acidic conditions. I’ve seen teams favor sulfuric acid as the catalyst to drive the esterification process to completion and strip out residual water, boosting yield. Scaling up means managing heat generation and careful distillation to purify the final product. The process highlights how vital temperature control and careful reagent ratios become—too little acid, and conversion slows; too much, and unwanted side products slip through purification. Industrial synthesis can require tweaks based on equipment, but the essential chemistry remains the same, just magnified to ton-scale output. The challenge always lies in keeping both conversion efficiency and downstream purification cost-effective.
Chemists value Methyl Toluene-4-Sulphonate for more than just connectivity; its methyl group anchors numerous substitution and elimination reactions. In one common reaction, nucleophiles displace the methyl sulfonate, opening routes to new ethers and amines used in pharmaceutical or agricultural projects. Some teams modify the sulfonate or methyl group to build custom-functionalized building blocks. Electrochemical studies sometimes examine the compound’s stability under reductive conditions, seeking either new reaction mechanisms or green chemistry upgrades. Where some reagents fumble under pressure, this sulfonate stands up, highlighting why organic chemists keep it close at hand. Mistakes in handling or reaction design can create bottlenecks, but experienced hands work around these with careful planning.
Supply chain paperwork and research articles list several names for Methyl Toluene-4-Sulphonate. Look for 4-Methylbenzenesulfonic acid methyl ester, Tosyl methyl ester, or even para-Toluenesulfonic acid methyl ester. These names all point to minor structure tweaks or toluene ring orientation. Mixing up nomenclature can snarl orders or trip up regulatory filings. The most cautious operators double-check both CAS numbers and structural diagrams before ordering or handling a new batch, avoiding costly mix-ups in synthesis planning or safety audit paperwork.
The handling of Methyl Toluene-4-Sulphonate prompts clear rules, not just because of safety codes but personal experience in busy labs. Direct contact can irritate eyes and skin, and inhalation of dust or vapors causes respiratory discomfort. Teams set up workspaces with airflow enclosures, wear well-fitted gloves and goggles, and keep emergency wash stations close. Shipping standards demand packaging robust enough to survive long journeys without leaking or breaking. Waste disposal follows regional hazardous chemical frameworks, and many sites audit their protocols to find improvement gaps before regulators catch them. Most incidents tracked back to simple lapses—rushed PPE use, sloppy labeling, or skips in air monitoring.
Pharmaceutical manufacturers rely on Methyl Toluene-4-Sulphonate as both a reagent and a protecting group source. Large-scale drug campaigns tap into its methylation capabilities, often during custom synthesis runs where speed and reliability decide profitability. Its role in dye production, especially during key steps to add or modify color-fast properties, extends to fibers and textiles. Specialty polymers and agricultural chemicals mark further domains, showing how a single synthetic intermediate branches out across industries. Deciding to use this compound can mean the difference between meeting a tight project deadline and months of retooling, all because process chemistry needs tools that don’t falter when scaled up.
Research groups keep exploring new catalysis strategies, hoping to trim waste and energy costs in Methyl Toluene-4-Sulphonate synthesis. Some focus on greener sulfonation reagents or solvent recovery loops, aiming for lower environmental impact. Analytical chemists review process impurities, trying to shave risk in pharma pipelines or boost batch consistency. Peer-reviewed studies document the results, flagged for anyone tracking data-driven process validation or innovation in green chemistry. Close partnerships between academia and industry mean some advances reach plant floors quickly. My time reviewing R&D portfolios showed that a compound with such diverse use has no shortage of bright minds pushing for improvement, often by combining old wisdom with novel tweaks.
Toxicology studies collect hard-won data showing moderate acute toxicity, especially in rodents, and the potential for skin and mucous membrane irritation. Chronic inhalation risks stay largely unstudied, so most sites stick with caution, limiting airborne exposures and setting routine monitoring schedules. Regulatory agencies pull from both animal studies and case reports to shape workplace safety recommendations. The data underline how quickly safety margins disappear if protocol slips, especially when handling reactive esters around heat or open vessels. Some work pushes into environmental toxicity, studying breakdown rates and persistence in soil or water. The evidence speaks plainly enough for anyone who’s seen teams manage spills or decontaminate workspaces.
Rising demand for targeted drug synthesis and green chemistry points to a growing role for Methyl Toluene-4-Sulphonate in years to come. Process optimization, especially for waste minimization and energy reduction, continues to pull both investment and regulatory attention. Manufacturers and research labs work to extend the compound’s use into emerging materials, like advanced electronics and specialty coatings, betting on versatility as product lines shift toward sustainability. Automation and AI-guided synthesis routes might soon redefine production, lowering barriers for smaller outfits without compromising product quality. For those of us watching the chemical landscape, the drive lies in making practical improvements that stick, building on a foundation secured by decades of hard work and stubborn commitment to safety and performance.
Methyl toluene-4-sulphonate might sound like something only a chemist would care about, but this chemical plays a solid, behind-the-scenes role in many corners of industry. I learned just how quietly powerful these specialty chemicals could be after working a few years with a team that supported chemical manufacturing in Southeast Asia. It’s not some household name, but walk through a factory or a research center, and you might spot its fingerprints.
This compound shows up in organic synthesis, which basically means it helps build other chemicals. It works like a middleman, passing along certain groups to new molecules so more complex structures can form. For anyone working in pharmaceuticals, that process turns out to be essential. Methyl toluene-4-sulphonate often helps create the “scaffolding” for active pharmaceutical ingredients (APIs). Reliable building blocks make the difference between a promising new drug and a failed experiment. The chemical acts as what’s called a methylating or tosylating agent. Scientists use it to add certain functional groups, shaping molecules with the right properties for treatments that the world depends on—think blood pressure pills, cancer treatments, or basic antibiotics.
Chemists working in the plastics or resins sector also value this compound. They use it to start or stop chain reactions that create specialized plastics. Modern electronics wouldn’t exist as they do without careful control over things like circuit boards and insulating materials; methyl toluene-4-sulphonate helps shape those materials at the molecular level. Some resins used in high-voltage transformers rely on modifications that this chemical can kick off. Without these, reliability drops and repairs cost more.
My own approach to working with any specialty chemical always puts data and safety first. Reading safety data sheets and talking to seasoned process engineers taught me the risks and the friction between profit and protection. Sulphonate compounds sometimes produce toxic byproducts, so the work site must manage everything properly. Several global manufacturers provide technical bulletins and transparent handling guidelines. Mistakes in storage or disposal lead to workplace injuries or local pollution, so hands-on knowledge and a culture of respect for chemicals matter a lot. The European Chemicals Agency and the US Environmental Protection Agency offer guidelines that responsible firms follow. Some require periodic training for everyone from managers down to technicians, covering not just storage but clean-up and emergency first-response scenarios.
As the world moves toward greener methods, chemists actively search for less hazardous alternatives. That doesn’t mean methyl toluene-4-sulphonate disappears overnight. Manufacturers invest in closed systems, regular air quality checks, and better waste treatment to keep both people and the environment safe. Technology keeps pushing safer protocols, but workers’ judgment and experience still matter most in a pinch. Regular audits, more transparent reporting, and investment in local communities can reduce the risks tied to chemical misuse and disposal.
Pharmaceuticals, specialty plastics, and modern electronics don’t just roll out of thin air. People on the ground, following solid science and responsible protocols, make these industries run safely and effectively. Methyl toluene-4-sulphonate stands as one piece in that larger puzzle, worth understanding for what it enables and for the commitment it demands from everyone involved.
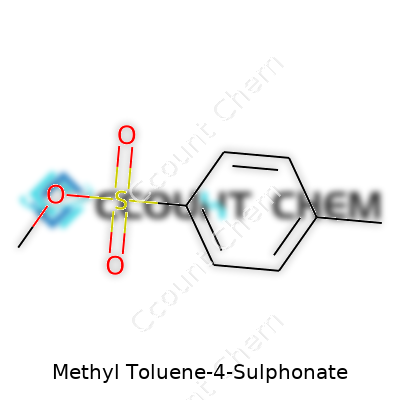
Plenty of topics in chemistry throw everyday people off balance, and organic compounds turn that confusion up a notch. Methyl toluene-4-sulphonate comes with a complex-sounding name, but for anyone who ever worked in a science lab—or sat through a well-taught chemistry lesson—the logic begins at the structure. Here’s how it lays out: you have a toluene base. That’s benzene with a methyl group at position one. Then, you toss in a sulphonate group, which attaches itself at position four (the para position). A methyl group also links up with the toluene’s sulphonate oxygen, and that’s how you get methyl toluene-4-sulphonate.
For the chemistry behind it, the formula stands as C8H10SO3. Breaking that down: eight carbons, ten hydrogens, one sulfur, and three oxygens. Every atom in that arrangement plays a job. The entire compound balances its properties between an aromatic ring, an electron-withdrawing sulphonate group, and a reactive methyl ester.
Some years back, I spent afternoons troubleshooting synthesis hurdles in a small chemical production lab. Any slip—using the wrong alkyl group or missing where to add a sulphonate—spelled wasted time and ruined product. Toluene derivatives like this play roles beyond benches and beakers. Industries rely on methyl toluene-4-sulphonate to transfer a sulfonyl group neatly onto other molecules. They serve as methylating agents, especially when chemists want to swap out a less reactive group with a methyl one without disrupting the whole molecule.
A compound with a clear-cut structure and reliable reactivity could mean efficient pharmaceutical research, cleaner specialties in dyes, and tighter control in polymers. A single misstep in identifying or preparing this chemical spells out not just disappointment, but health risks and compliance issues. Strict attention to formulas like C8H10SO3 keeps both production and application safer and more predictable.
Getting the chemical formula spot-on matters for a bigger reason than nerdy pride. Chemicals move across borders and through hands. One slip in documentation—listing C8H10SO3 as something else—carries real consequences. Factories run into regulatory snags. Lab workers misjudge hazards. Researchers waste time and budget chasing incorrect leads. This isn’t just a matter of memorizing a string of letters and numbers.
History has lessons here. In my own time on the floor, I saw how taking shortcuts or guessing at a chemical’s makeup left entire batches unsellable. Getting formulas right won’t fix every problem that haunts the industry, but it clears up one particularly stubborn part of production confusion. Open sharing of reliable specs, from research journals to suppliers, pushes everyone forward. On the safety side, knowing exactly what sits in a drum or flask makes storage, transport, and emergency protocols easier to plan.
To keep missteps rare, digital resources offer real help. Databases like PubChem or ChemSpider catalogue these formulas in plain sight. In my experience, double-checking figures through several sources, plus talking with a company’s technical team, heads off mix-ups. Manufacturers can add QR codes or digital tracking along the supply chain to keep everyone honest.
For anyone tackling chemical formulas day in and day out, don’t shrug off the fundamentals. The right answer—C8H10SO3 for methyl toluene-4-sulphonate—saves time and builds safer, more effective work down the line.
Methyl Toluene-4-Sulphonate doesn’t roll off the tongue, and it rarely shows up in common conversation. Yet this chemical plays a role in various industrial processes, often as a chemical intermediate or catalyst. Before dismissing it as another complicated ingredient used in manufacturing, it’s worth digging deeper into whether it poses hazards that ordinary people, not just factory workers, ought to be concerned about.
Many safety data sheets rank Methyl Toluene-4-Sulphonate as harmful if swallowed, inhaled, or allowed to contact skin or eyes. Exposure in high quantities could cause irritation or even more serious effects like respiratory distress. This tracks with what many aromatic sulfonates produce: discomfort, short-term reactions, and risks that ramp up where exposure is heavy and longtime, like workshops or chemical plants without proper gear.
The Environmental Protection Agency and OSHA don’t list it among the worst offenders—the chemicals that call for specialized hazmat teams at every turn. It still deserves respect. My own foray in a university lab emphasized this. After a small accidental spill, my professor insisted on immediate clean-up, gloves, goggles, and a thorough scrubbing of every surface the compound touched. Not because we expected tragedy, but because it’s easy to underestimate what exposure compounds over time, and the body doesn’t shake off chemical irritation as quickly as it does spilled coffee.
People assume the biggest risks in chemistry sit in products with loud, clear warnings. Everyday reality reminds us many chemicals with unfamiliar names can slip into the workspace quietly, especially in places making dyes, resins, or specialty coatings. Factory floors, mixing rooms, and packaging lines—folks there develop coughs or rashes and miss the connection to what’s in their hands all day. Letting that slide can ramp up healthcare costs, lost time, and frustrated workers. Even short-term skin or eye trouble messes with productivity and morale.
Pushing toward greater safety means treating every unfamiliar chemical as worthy of caution. Gloves, goggles, plenty of ventilation—these aren’t just bureaucratic rules, they’re the basic armor that keeps workers healthy. Clear labelling, safety briefings, quick cleanup kits—companies investing in these build loyalty and cut long-term costs. No one should have to gamble on “rare” allergic reactions or the chance of chemical burns, no matter how rare they claim to be. In my time on a plant tour, one worker told me the only reason he felt comfortable was because his team had recently overhauled their training, turning vague warnings into step-by-step routines anyone could follow under pressure.
Switching to greener, less hazardous alternatives is an option for some. Using digital records to track near-misses and exposures means problems don’t disappear under the radar. Through real stories and evidence, it becomes clear: safety isn’t about chasing every invisible hazard, but about equipping communities—workplaces, schools, labs—to treat every chemical with the seriousness it might deserve. Methyl Toluene-4-Sulphonate isn’t a household name, but the lessons it prompts ring true for every hidden bottle tucked on a shelf and every routine we take for granted.
Smart storage plays a big role in handling chemicals like methyl toluene-4-sulphonate. This compound brings value to the chemical industry, used in pharmaceuticals and dyes. In daily work at manufacturing sites and distribution points, careless handling of this powder risks safety gaps and puts staff health on the line. Chemical safety, I’ve learned, starts right at the warehouse door.
Contact with air and moisture triggers slow decomposition or even dangerous chemical reactions for methyl toluene-4-sulphonate. Fumes can irritate eyes, skin, or the respiratory system. I’ve watched spills create headaches not only for safety teams but for regular workers just doing their jobs. People want to work in a space where fumes and dust don’t become a regular concern, and accidents rarely happen by chance.
Place drums or bags in a cool, well-ventilated spot, far from sunlight or heat sources. Methyl toluene-4-sulphonate reacts badly to humidity. Use tightly sealed, corrosion-resistant containers—think high-density polyethylene or steel with the right lining. When I walked through chemical warehouses over the years, staff always checked lids for even the smallest cracks. A single leak can make a whole row of storage hazardous.
Storing away from acids, oxidizing agents, and sources of ignition makes sense. Combustible dust builds up if storage gets sloppy, leading to fire risks. Clear labeling limits confusion, helping everyone—from new staff to delivery drivers—know what they’re handling. I’ve seen time and again that labeling cuts down on mix-ups and stops risky shortcuts.
Lab coats, gloves, goggles, and proper air filtration take the edge off routine handling. Respirators might not make daily tasks easy, but they make a difference in high-dust zones. I’ve seen how respiratory shields and fume hoods go from “nice-to-have” to “lifesaver” in places where powder moves in large volumes.
Training stands out as an underrated piece. A warehouse with posters on spill drills and emergency contacts creates muscle memory. People rely on habits in moments of panic, and drills build those habits. Every shop floor I worked on kept a spill kit within arm’s reach; quick action always beats emergency room visits.
Temperature checks and humidity sensors help spot creeping dangers. Modern storage spaces use alarms tied to set thresholds. These tools aren’t overkill—they stop a quiet buildup from becoming a front-page headline. Records of inspections provide proof for management and auditors that nothing falls through the cracks.
Old habits die hard. Some facilities cut corners when no one’s watching. Audits and regular inspections nudge everyone back on track, reinforcing that safety is more than box-ticking. Smart companies keep emergency plans up-to-date and make their first aid kits easy to find—because the simple steps save lives.
A lot of people skip the small print about chemical safety. In my own lab days, I saw more than a few co-workers jump right in, gloves off, thinking nothing bad would come from a quick pour. It only took once to learn. Methyl Toluene-4-Sulphonate (MTS) comes with its own set of warnings for a reason. Even if a substance feels ordinary, it doesn’t mean a person can handle it like kitchen sugar. If anything, just about every industrial solvent or intermediate can turn a routine task into a trip to the emergency room without the right precautions.
The sting of MTS on the skin is not something most forget. It’s not only about irritation—this compound has the potential to cause longer lasting harm or trigger allergic reactions. Gloves stand between the chemical and your hands. Nitrile tends to win over latex for MTS; not all gloves hold up the same. Goggles do more than fog up your vision: a single splash can mess up an eye, and nobody should leave eyesight to chance. In tough spots, where splashes seem likely, a full face shield can become as important as your shoes.
Lungs can’t back out of a fight with dusts or fumes the way hands can. When MTS gets airborne, it finds its way into a person’s airway easily. My own mistake once—skipping a proper respirator—led to a sore throat lasting days. Good engineering controls like fume hoods do the heavy lifting, drawing vapors away before anyone breathes them in. If you smell something odd, or see dust floating around, trust your nose—ventilation likely isn’t up to speed.
Nobody plans for a spill, but they always seem to happen right when the day gets busy. An open bottle, quick movement, and suddenly MTS covers the bench. Instead of just grabbing a paper towel, stop and grab a chemical spill kit. These kits often include absorbent materials that actually neutralize the compound, rather than spreading it around. Clear the area, warn your colleagues, and consult the material safety data sheet for proper disposal—skip the guesswork. Having these steps in place means everyone knows what to do, not just the person closest to the bottle.
Storing MTS goes beyond tossing it on the shelf with other reagents. It belongs in a cool, dry spot, away from both acids and bases, because mismanagement risks unexpected reactions. Labeling matters—no one likes a guessing game when rummaging through bottles. Proper training puts these rules into action. Every time new staff joins, or an old friend gets rusty, a run-through of real hands-on practice beats any long lecture. I’ve seen workplace cultures change dramatically just from shifting attitudes about routine safety talks.
Most of what people call best practices come from real accidents, not just regulations. A single moment of care—donning full gear, cleaning up fast, asking for help—carries more weight than a shelf full of manuals. MTS isn’t a villain on its own, but overlooking basic steps can make any chemical a real hazard. The simple truth is, habits stick. Building good ones at the bench isn’t about getting an award, but about heading home healthy at the end of the day.
| Names | |
| Preferred IUPAC name | methyl 4-methylbenzenesulfonate |
| Other names |
Tosylmethane p-Toluenesulfonic acid methyl ester Methyl p-toluenesulfonate Methyl 4-methylbenzenesulfonate |
| Pronunciation | /ˈmɛθɪl tɒˈljuːiːn fɔːr sʌlˈfəʊneɪt/ |
| Identifiers | |
| CAS Number | 80-48-8 |
| 3D model (JSmol) | `CC1=CC=C(C)C=C1S(=O)(=O)O` |
| Beilstein Reference | 1720801 |
| ChEBI | CHEBI:51264 |
| ChEMBL | CHEMBL46184 |
| ChemSpider | 110868 |
| DrugBank | DB14082 |
| ECHA InfoCard | 100.019.591 |
| EC Number | 202-576-2 |
| Gmelin Reference | 8325 |
| KEGG | C19268 |
| MeSH | D008755 |
| PubChem CID | 8552 |
| RTECS number | WN5250000 |
| UNII | DS6P49A4F9 |
| UN number | “UN2811” |
| Properties | |
| Chemical formula | C8H10O3S |
| Molar mass | 186.22 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.18 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.90 |
| Vapor pressure | 0.003 hPa (20°C) |
| Acidity (pKa) | -2.8 |
| Basicity (pKb) | 7.86 |
| Magnetic susceptibility (χ) | -54.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.538 |
| Viscosity | 12-16 cP |
| Dipole moment | 3.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 332.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -595.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6068.7 kJ/mol |
| Pharmacology | |
| ATC code | V03AB37 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H225, H302, H315, H318, H332, H335 |
| Precautionary statements | P210, P261, P264, P271, P280, P301+P312, P304+P340, P305+P351+P338, P312, P337+P313, P403+P233, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 120°C |
| Autoignition temperature | 450°C |
| Lethal dose or concentration | Lethal dose or concentration (string): LD50 oral rat: 1750 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 1750 mg/kg |
| NIOSH | WX8575000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Methyl Toluene-4-Sulphonate: Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) for Methyl Toluene-4-Sulphonate: Not established |
| Related compounds | |
| Related compounds |
Toluene-4-sulfonic acid Toluene Methyl tosylate Benzenesulfonic acid Sulfanilic acid |