Methanesulphonic Acid (MSA) did not always draw much attention. Early chemists overlooked sulfonic acids compared to their more dramatic cousins—strong mineral acids. By the middle of the last century, research focus sharpened. Scientists in France and Germany discovered sufonic acids could do the heavy lifting mineral acids handled, minus some drawbacks. Over decades, curiosity about MSA’s clean reactions, low volatility, and straightforward synthetic routes turned it into a workhorse of organic chemistry and specialty manufacturing. The industry grew rapidly as people started looking for safer acids that delivered results without the tightrope walk of traditional choices like sulfuric or hydrochloric acid.
Industry recognizes methanesulphonic acid as a colorless liquid that packs a punch. Experts use it as an acid catalyst and a sulfonating agent. The quality that sticks out is its strength as an acid—it easily compares to sulfuric acid in terms of dissociation without releasing choking fumes. This gives chemists room to breathe, both literally and figuratively, especially in labs and enclosed setups. Unlike sulfuric acid, which clashes with moisture and turns bottlenecks rusty, MSA stays manageable and consistent even after repeated exposure to air.
MSA’s high boiling point, near 167°C, tells you it sticks around even under heat. It comes with a melting point near -51°C, meaning it works fine in frigid conditions. Its density, hovering close to 1.48 g/cm³, says it’s heavier than water but easy to pour and measure in bulk. Throw it in water and it dissolves completely—no stirring for hours or awkward stratifying. The clear, nearly odorless nature makes it less irritating to handle compared to other acids. Its molecular structure, one methyl group attached to the robust SO3H, keeps it reactive yet predictable.
Every jug or drum shipping out of factories comes with strict labeling. Standard MSA on the market often tops 99% purity, making it suitable for electronics, pharmaceuticals, and high-purity applications. Quality control teams pull out titration kits, melting point apparatus, and chromatographs to check for sulfate, heavy metals, and even color. Reliable producers always provide batch numbers and lot info, both for traceability and for peace of mind should anything go sideways during use. Labels include hazard pictograms showing both the corrosive and environmental warning signs, matching globally recognized GHS standards.
Straightforward methods dominate in industry, starting with methyl mercaptan or dimethyl sulfide, oxidized with agents like chlorine or hydrogen peroxide. Labs often stick with easier-to-control routes, reacting methyl iodide with sodium bisulfite under tight conditions. No need for rare catalysts or exotic procedures. The often-cited “clean” nature of MSA’s synthesis means less waste and safer handling. Years ago, people worried about chloride byproducts or noxious gases, but the latest processes reduce environment-threatening emissions far below regulatory limits.
Anyone who’s spent time in a chemical plant knows MSA brings a lot to the table. It acts as a super-strong acid, outgunning acetic and formic acids for most tasks. Acylations, esterifications, and alkylation reactions often swap in MSA for more aggressive acids thanks to its no-fuss, no-smoke character. It readily forms methanesulfonates with alcohols and amines. Chemists even use it in electroplating baths to help lay down smooth, shiny metal layers. People are experimenting with derivatives like methanesulfonic anhydride, which brings a punch of reactivity for certain organic transformations.
Chemistry textbooks and industrial catalogs reference methanesulphonic acid under various aliases. You’ll see it written as MSA, methylsulfonic acid, and methane sulfonic acid. Trade names are everywhere, each manufacturer eager to stake a claim in the market—look for “Mesylic acid” or similar branding. It’s not rare to spot other-language variants or abbreviations in import and export documents, so anyone moving shipments internationally keeps a close eye on synonym lists.
Any acid can burn, but MSA’s lower volatility makes it less likely to splash or aerosolize into an instant health hazard. Still, contact with skin or eyes causes deep burns. A solid respect for gloves, face shields, and fume hoods pays off here. Modern safety sheets stress its reactivity with bases and some metals, so no one puts it near containers that might corrode. Cleanup procedures draw on industry best-practices—dilute with large volumes of water, neutralize with sodium bicarbonate, and always, always ventilate. Storage in plastic drums, away from direct sunlight and temperature swings, keeps the stuff stable for months.
Ask professionals in electronics why MSA matters, they’ll say it makes copper plating not only more efficient but more reliable. Pharmaceutical labs count on it to shape complex molecules where other acids would overly scorch delicate building blocks. Drug synthesis steps involving protecting groups or ring closures often benefit from MSA’s non-oxidizing nature. The battery industry now looks at MSA-based electrolytes for next-generation zinc batteries due to improved safety and longer lifetimes. Rust remover manufacturers like its strength without tossing hazardous fumes into workshops.
Universities and labs are constantly testing MSA. In green chemistry circles, researchers compare it to classic acids in catalytic cycles, keeping records of lower emissions and milder reaction conditions. Scientists looking to push limits try MSA in flow chemistry, swapping batch reactors for safer, continuous processes. Teams in electrochemistry play with methanesulphonate salts to improve conductivity and electrode stability. Custom polymer groups focus on MSA’s role as a polymerization initiator, targeting adhesives and specialty coatings with better resistance to heat and chemicals.
Early studies suggested MSA’s toxicity fell well below that of many mineral acids. Acute contact still draws warnings—skin contact burns, inhalation irritates, and ingestion causes serious harm. Detailed animal studies found no pronounced carcinogenicity at industrial exposure levels, offering relief to regulatory boards and occupational safety teams. Fish and aquatic invertebrates handle low levels with minimal distress, though ethics demand responsible disposal and treatment. Ongoing work tracks breakdown in the environment, aiming to confirm rapid decomposition and low persistence—important traits for green chemistry advocates.
MSA stands at the crossroads of safer chemistry and higher performance. Battery makers chase its promise for extending cycle life while keeping fire risks lower than other electrolytes. Water treatment facilities are running pilot setups with MSA-based cleaning routines to cut chlorine emissions. Looking into fine chemicals, developers want to exploit its clean, strong acid punch to swap out older, dirtier reagents. With tight regulation closing in on many acids with high volatility or known hazards, MSA’s footprint across industries continues to spread. Ongoing studies target bio-based feedstocks and circular production, aiming to close the loop on raw material sourcing. As more people demand better performance with lower risk, methanesulphonic acid looks well-positioned for breakthroughs.
Methanesulphonic acid draws a lot of attention in chemical industries. Its strong acidity and low toxicity give it an edge over other strong acids like sulfuric or hydrochloric acid. This combination sets it apart, both in terms of performance and safer handling. In my time working in a laboratory setting, switching from harsher acids to methanesulphonic acid brought a sigh of relief among lab techs. It created a less hazardous environment, lowered the risk of accidents, and simplified disposal procedures.
Pharmaceutical production runs smoother with methanesulphonic acid around. Many drug molecules rely on this acid during synthesis. Its predictability and reliability play a huge role in reactions, often helping chemists speed up production without compromising quality. My old lab used it to make antibiotics where clean reaction pathways meant fewer side products, saving hours in purification. Reliable acids like this one support the kind of consistency regulators demand and patients need.
The electronics industry continues to grapple with the needs of miniaturization and higher performance. Methanesulphonic acid supports metal plating without creating noxious fumes or the high levels of waste typical of other acids. For anyone who’s stood over an open electroplating bath, the difference is night and day. After the switch, I noticed fewer headaches and stronger worker retention. Companies using this acid in tin and gold plating benefit from a smaller environmental footprint and higher deposition rates, translating to cost savings on waste management and ventilation.
Anyone who’s faced calcium build-up in pipes knows the importance of a powerful but manageable acid. Methanesulphonic acid strips away mineral scale and rust efficiently, but what makes it stand out is its readiness to rinse off and lower chance of damaging the underlying metal. For large building maintenance or car repair workshops, the shift to methanesulphonic acid cuts down on pitting, extending the lifespans of valuable parts and plumbing. End-users appreciate fewer harmful fumes and faster recycling of cleaned equipment.
Every time a business makes decisions about chemicals, the long-term effects on groundwater, air, and soil come into play. Methanesulphonic acid breaks down more easily and poses a lower threat to waterways compared to many industrial acids. Responsible handling matters: companies that invest in proper storage and neutralization see fewer spills, face fewer fines, and contribute to less long-term contamination. Regulations around its transport and use set a necessary standard, but from experience, ongoing education in workplaces remains critical. Staff training and clear labeling go farther than mandated rules alone.
Choosing methanesulphonic acid opens more doors for greener chemistry. As demand rises for sustainable production, businesses look for options that keep efficiency high and danger lower. The acid’s growing reputation rises on its blend of power and gentleness. The move toward safer operations isn’t just about regulation—it reflects a larger shift in values within manufacturing and research. Communities, workers, and the environment all gain when industries make that change thoughtfully.
Methanesulphonic acid (MSA), a colorless liquid that brings muscle to chemical reactions, holds a quiet but powerful place across several industries. Whether you’re out in a plating workshop, tinkering with electronics, or working in a pharmaceutical lab, you’ve probably bumped into this acid. The real question many face though—what’s in the bottle, exactly? Concentration and purity matter, and not only to chemical engineers.
In most industrial use, MSA comes in a concentration of around 70% by weight. That’s pretty dense. Some suppliers also offer grades ranging from 99% to above 99.5%, but 70% lands as the market favorite for general processing. The water content helps give flexibility—you can dilute for fine-tuned applications or use out-of-the-bottle for tough reactions.
Purity sits right at the heart of safety and reliability. Folks working in pharmaceuticals or electronics don’t gamble with contaminants. Any stray metal ions or organic leftovers mess with electroplating baths or react with other ingredients. High purity—usually above 99%—means less risk for side reactions. In my old university lab, we’d always reach for higher purity MSA when prepping reaction media, since unknown impurities often spelled a wasted afternoon and a sticky mess in the glassware.
I’ve seen chemists and lab techs swear off cheaper, low-grade acids after a single contaminated batch. Even trace sulfuric acid, leftover from manufacturing, can corrode equipment faster or lead to unwanted by-products. Pharmaceutical companies won’t touch acids below 99% purity for good reason. The downstream costs of impurities—poor yields, cleaning, tainted drugs—far outweigh a slight upcharge for pure product.
Beyond pharmaceuticals, high-purity MSA means tighter control over plating baths for circuit boards or battery components. It extends equipment life, saves hours in quality control, and keeps environmental compliance simpler down the line. Customers—engineers, lab managers, manufacturers—notice when a batch isn’t consistent. Reputation and repeat business stick to suppliers who deliver predictable chemistry.
Labels can say a lot but true confidence comes from transparency. Good suppliers support their purity claims with detailed certificates of analysis (CoA) from independent labs. These show heavy metal content, water levels, organic contaminants, and more. If a supplier dodges those requests or hides behind generic data sheets, buyers usually walk.
Relying on reputable sources keeps safety standards high. Working with MSA doesn’t offer much room for error—splashes burn, vapors can irritate, and careless storage leads to broken containers. High purity reduces odd reactions and unpredictability, helping firms build strong safety records and meet regional regulations from bodies such as the European Chemicals Agency (ECHA) or the US Environmental Protection Agency (EPA).
Buying solid, high-concentration MSA isn’t just for labs with deep pockets. Even small workshops and startups benefit from peace of mind and improved yields when they invest in the good stuff. If purity dips or batches change, it usually points to supply chain shortcuts or weak oversight. Vetting suppliers, requesting CoAs, and joining a network that shares honest experiences all help dodge setbacks.
Transparent labeling, responsible sourcing, and ongoing feedback keep the focus on quality instead of price-wars. Cheaper acids can wreck equipment, harm workers, and drag out project timelines. Reliable MSA brings better outcomes, happier crews, and fewer headaches along the way.
You’ll find methanesulphonic acid cropping up in a bunch of applications, from electroplating to pharmaceuticals. In the chemical industry, it’s pretty valuable as an acid catalyst, especially since it has low vapor pressure and skips out on producing that choking acidic mist that some other acids throw in everyone’s faces. That seems like a safety bonus at first glance. Still, if safety advisers put methanesulphonic acid on their radar, it’s not because it’s “harmless.” People working with methanesulphonic acid ought to recognize that hazards still come with the territory, even if this acid behaves differently from sulfuric or hydrochloric acid.
Looking at the facts, methanesulphonic acid holds its title as a strong acid. It scores low on the pH scale. If it spills on skin or splashes into eyes, that burn you feel is real. I remember a friend at a plating plant who brushed a sleeve soaked in diluted methanesulphonic acid against his wrist. His skin turned red almost instantly. At higher concentrations, this acid chews through organic tissue—imagine the situation with eyes or open wounds. I’ve seen lab benches after a careless spill; the surface bubbled and discolored in no time. That’s pure corrosive action, no question.
Corrosivity extends to metals too. Methanesulphonic acid gets used in cleaning pipes and descaling equipment, proving it dissolves tough mineral buildup. But switch the context, and think of a worker’s poorly fastened metal watch or belt buckle, and you’ve got a recipe for ruined equipment and broken safety gear. Metal fittings on pumps and tanks need regular checks for pitting and leaks. So, while it doesn’t fume aggressively, it can quietly cut through barriers over time.
Acute exposure causes immediate problems—skin burns, eye injuries, throat pain if inhaled as an aerosol. Swallowing methanesulphonic acid is a medical emergency. Long-term exposure data lags behind some better-studied acids, but the corrosive effects are well documented. The European Chemicals Agency classifies it as corrosive, so regulatory bodies don’t downplay these risks.
People might think “organic acid" means “softer touch.” Reality checks come quick. Methanesulphonic acid’s ability to break chemical bonds, denature proteins, and eat away at surfaces puts it on par with other acids known for causing harm.
Working around methanesulphonic acid demands respect for personal protective equipment. Lab goggles, chemical-resistant gloves, PVC aprons—these stop burns before they start. In smaller operations, I’ve watched managers cut corners, swapping out purpose-designed gloves for the cheapest pair on the shelf. The consequence always winds up with someone losing time to hospital visits. Engineering controls matter too. Eyewash stations, fume extractors, and spill containment gear reduce risk drastically.
Training is another line of defense. Too often, people trust their instincts or lean on old habits. That doesn’t work with a strong acid. Regular, clear instructions prevent mistakes. Team briefings and updated safety sheets, not just a binder gathering dust on a back shelf, make a big difference. OSHA and international chemical safety standards set strong guidelines. Following them keeps real people safe, not just company statistics looking good.
It’s tempting to see methanesulphonic acid as “modern” or “environmentally friendly” compared to nastier options. Still, calling it safe without thinking about its corrosive bite costs too much in the long run. Handling it carefully, respecting the hazard, and building strong safety routines keep operations running smoothly. A clean record and healthy staff prove that giving corrosive acids the respect they deserve pays off every time.
Walk into any lab that deals with powerful acids and you pick up the same lesson quickly: respect every chemical, especially the ones that can bite back. Methanesulphonic acid, or MSA, packs a punch as a strong acid, but its reputation for being “less volatile” than others often lulls folks into a false sense of comfort. It’s clear and almost odorless—easy to overlook. The truth: one careless splash or a container failure can create emergencies you won’t forget.
A storage area counts as the lab’s fortress. Methanesulphonic acid can chew through weak plastics and corrode metal in no time. Shelving built of high-density polyethylene, polypropylene, or coated steel prevents this. I’ve seen old shelving collapse because someone thought “it’ll hold just fine.” It didn’t. Acid storage cabinets marked for corrosives reduce chances of reactions or leaks. For anyone sharing a stash of chemicals, strict segregation rules matter. No acid should sit next to bases—mixing those by accident produces toxic fumes and plenty of regret.
Wearing goggles and gloves is the most basic step, but plenty of experienced folks slip up, especially when rushed. Nitrile and neoprene gloves do the trick much better than latex, which gets eaten away fast. A full face shield makes sense if there’s any risk of splashing. I keep clean lab coats and aprons by the door and make sure they’re not worn outside, to avoid dragging acid or residue everywhere.
It’s easy to underestimate how a splash or fume can build up inside closed spaces. Proper ventilation pulls vapors away from breathing zones. I’ve worked in an old facility where a lack of airflow resulted in nose and throat irritation—something people just “got used to,” which is never okay. Fume hoods or localized extractors keep everyone safer.
Some people trust memory for what’s inside a bottle. One mix-up can hurt people or damage gear. Clear labels—big ones—are mandatory on every single bottle and transfer container. MSDS sheets should always stay in easy reach, and everyone should know where spill kits and eye washes are located.
I’ve never seen careful training go to waste. Staff members who walk through safe handling procedures, spill clean-ups, and emergency moves feel much more confident. They don’t freeze in a real accident. Regular refreshers keep new and old hands up to speed.
Even with trust in protocols, it’s tempting to cut corners when things get busy. A rushed transfer, a forgotten glove swap, a container left uncapped—these habits lead straight to burns, inhalation risks, or workplace contamination. A true safety culture relies on stopping unsafe shortcuts, with supervisors open to hearing concerns.
Each lab I’ve spent time in that avoids accidents takes storage and handling seriously. They invest in the right hardware, keep the right supplies on hand, train people, and never rely on luck. Methanesulphonic acid won’t cause drama unless mistakes happen, and it never forgives carelessness. Smart storage, correct gear, clear training, and genuine attention to detail create the safest possible workspace.
Methanesulphonic acid attracts interest across a broad sweep of industries, from electronics and pharmaceuticals to metal processing. This strong acid gets the job done in cleaning, etching, and chemical synthesis. Yet, there’s a practical side to handling methanesulphonic acid—choosing a package size that matches both need and safety.
Most companies dealing in chemicals focus on streamlining storage, transport, and on-site handling. The major packaging sizes for methanesulphonic acid reflect this. Smaller amounts, such as 500 mL and 1-liter bottles, suit research labs running bench-scale experiments. As demand grows in electroplating or larger cleaning jobs, suppliers step up with 5-liter and 25-liter containers.
Bulk users want even more, since refilling over and over slows progress and adds risk. Drums—usually 200 liters—step in as the mainstay for factories. These drums are fairly standard, and forklifts move them easily on shop floors or into storage areas. Tanker trucks or intermediate bulk containers (IBCs) push volume even higher. With IBCs, one tote can move 1,000 liters at a time, and deliveries can avoid the constant drum swapping that slows bulk operations.
Choosing a size isn’t just about convenience. Methanesulphonic acid reacts readily and demands respect in handling. Small bottles cut accident risk in laboratories and remove the need for extra equipment. That came clear during my time in a university chem lab, where handling bulk acid was never even discussed—small bottles were the rule.
On a production floor, labs can’t keep up with demand, and a steady supply is vital. At a friend’s specialty coatings business, they moved from 25-liter cans to 200-liter drums, cutting costs and downtime. Tracking acid levels became easier, and they reduced leaks by limiting transfers between containers. Less transferring meant safer working conditions and fewer spills—a detail supported by safety data from the U.S. Chemical Safety Board, which connects many chemical injuries to frequent material handling.
Transport rules keep both people and the environment safer. Packing methanesulphonic acid in leak-proof HDPE drums or IBCs matches both regulations and plain caution. Even the best can packaging should come with secondary containment—trays, bunds, or spill pallets—especially for drums and totes. Mishandling or inadequate packaging leads straight to EPA violations and cleanup headaches, as seen in some high-profile plant accidents.
Some suppliers do offer specialized packages, like lined steel drums, for customers with tougher or unique storage needs, but plastic containers remain the norm. Regular users should look for UN certification stamps or local chemical transport approvals, since these give a solid layer of reassurance about sturdiness.
There’s room to improve how acids get distributed. Dripless connectors and direct-from-drum dispensing systems already cut down on exposure. Reusable IBCs help knock down both waste and shipping costs, but users need discipline in cleaning and maintaining them. More suppliers now offer packaging returns, a step toward shrinking hazardous waste, which grows as chemical demand swells. Programs like ChemCycling promote reuse and responsible disposal, slowly shifting industry practices.
In short, picking a package size isn’t just a box on a form; it shapes how safely, efficiently, and responsibly companies use methanesulphonic acid. Matching size with need, experience, and practical safeguards can make a real-world difference in the lab, in the plant, and beyond.
| Names | |
| Preferred IUPAC name | Methanesulfonic acid |
| Other names |
Methanesulfonic acid MSA Methanesulfonate Methansulphonic acid Mesylic acid |
| Pronunciation | /ˌmɛ.θeɪn.sʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 75-75-2 |
| Beilstein Reference | 1713883 |
| ChEBI | CHEBI:41495 |
| ChEMBL | CHEMBL1377 |
| ChemSpider | 7557 |
| DrugBank | DB01968 |
| ECHA InfoCard | 03a3eafc-d3ad-4bb8-bf03-8145a7c15661 |
| EC Number | 200-898-6 |
| Gmelin Reference | 6078 |
| KEGG | C01033 |
| MeSH | D008740 |
| PubChem CID | 622 |
| RTECS number | PV7875000 |
| UNII | WHA5RIR98D |
| UN number | UN3265 |
| Properties | |
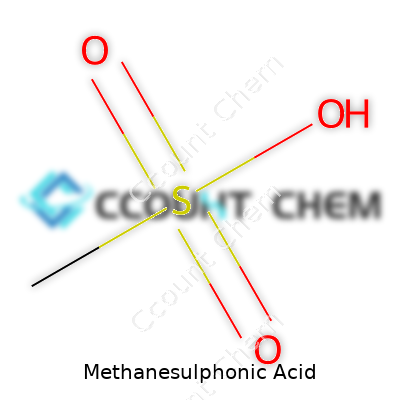
| Chemical formula | CH4O3S |
| Molar mass | 96.11 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 1.48 g/cm³ |
| Solubility in water | Miscible |
| log P | -2.05 |
| Vapor pressure | 14 hPa (20°C) |
| Acidity (pKa) | −1.9 |
| Basicity (pKb) | -2.9 |
| Magnetic susceptibility (χ) | -43.3 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.333 |
| Viscosity | 10 mPa·s |
| Dipole moment | 1.41 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 117.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -748.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -726 kJ/mol |
| Pharmacology | |
| ATC code | A16AX15 |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed, may cause respiratory irritation |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05 |
| Signal word | Danger |
| Hazard statements | H290, H314 |
| Precautionary statements | P234, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P363, P405, P501 |
| NFPA 704 (fire diamond) | 3-0-2-Acidos |
| Autoignition temperature | Autoignition temperature: 440 °C |
| Lethal dose or concentration | LD50 (oral, rat): 1600 mg/kg |
| LD50 (median dose) | LD50 (oral/rat): 1600 mg/kg |
| NIOSH | WSH19750 |
| PEL (Permissible) | 1 mg/m³ |
| REL (Recommended) | 1 ppm |
| IDLH (Immediate danger) | 100 mg/m³ |
| Related compounds | |
| Related compounds |
Methanesulphonate Methylsulfonylmethane Ethanesulfonic acid Sulfuric acid Trifluoromethanesulfonic acid Benzenesulfonic acid |