Chemistry laboratories in the nineteenth century buzzed with new discoveries, many led by curiosity about aromatic compounds. M-Xylenesulfonic acid sodium salt comes from a lineage of chemical exploration dating back to the early developments in sulfonation chemistry. Industrial demands pushed chemists to introduce sulfonic groups into aromatic rings, paving the way for a family of sulfonated derivatives. M-xylene, drawn from coal tar distillation and later from petroleum sources, found new meaning when researchers discovered sulfonation could enhance solubility and reactivity. Sodium salt derivatives like this one quickly moved from bench work to larger manufacturing thanks to textile dye advancements and detergent chemistry. Lab notebooks from those early days show the direct, sometimes messy, path of innovation. I’ve seen old process records where tweaks in sulfonation temperature led to dramatic gains in product yield, reflecting the hands-on, iterative nature of discovery rather than tidy textbook progress.
M-Xylenesulfonic acid sodium salt presents as a granular or crystalline powder, usually shining in shades of white or cream. Chemically, it’s the product of adding a sulfonic acid group to the meta position of xylene, neutralized with sodium hydroxide. The sodium salt form delivers much higher water solubility than its sulfonic acid counterpart. Its melting point, often falling in the 280–300°C range, stands out, resisting thermal decomposition under usual processing conditions. This stability and reliable purity, when specified above 98%, matter greatly in batch processing and analytics. Analytical data sheets, often several pages long, focus on sodium content, overall assay, moisture, and inorganic residue. Storage instructions stress sealed containers and dry, cool conditions. Every time I’ve had to inventory or check quality on this salt, straightforward parameters like these shaped purchasing and laboratory decisions, with trace impurities sometimes derailing synthesis or fouling up chromatography.
Manufacturing rarely stops evolving. Classic preparation involves direct sulfonation of m-xylene with oleum or concentrated sulfuric acid, monitoring temperature tightly to favor mono-sulfonation. Sulfonic acid conversion to the sodium salt runs through neutralization using sodium carbonate or sodium hydroxide. Filtration, washing, and careful crystallization follow—steps that show how process choices ripple down the production line. Process optimization teams earn their keep figuring out waste-stream minimization and energy savings without sacrificing purity. In graduate labs, this reaction sequence can demonstrate how to balance exothermic reactions and still walk away unscathed with good yield. Small errors in neutralization increase salt impurities or leave behind reactive acid, which I’ve seen lead to ruined catalyst runs or hazardous byproducts.
Reactively, the sulfonic group transforms how chemists interact with the xylene core. Nucleophilic substitution attaches other functional groups, and the sodium counterion means ready-to-use aqueous solutions. M-Xylenesulfonic acid sodium salt withstands much that destroys lesser organics—strong acids, bases, and oxidation—thanks to that aromatic base. Still, like other aromatic sulfonates, it can act as a leaving group under certain catalyzed scenarios, providing a springboard for synthetic transformation. I’ve watched teams exploit this reactivity to craft fluorescent dyes or pharmaceutical intermediates, using the predictable behavior of the sulfonate to anchor larger molecular frameworks. Those working on structure-reactivity relationships see this compound as a reliable test case for sulfonic group chemistry.
Naming conventions often travel with culture and language. Beyond the systematic “sodium m-xylenesulfonate,” suppliers sometimes list “meta-xylene sodium sulfonate” or even “sodium meta-xylenesulfonic acid.” CAS registry numbers serve as the true identifiers, slicing through any confusion linked to regional spelling or translation. Once, a supplier issue almost caused shipment mistakes for us, since the same material arrived under three synonymous titles. Product tracking software needs every synonym cross-referenced, or warehouse staff can end up with surplus stock or double-filled orders. Careful attention to labeling reduces risk of costly mix-ups, especially for multinational lab operations or global manufacturers.
Lab safety guides treat m-xylenesulfonic acid sodium salt as an irritant, which feels manageable compared to nastier chemical cousins. Direct exposure can cause skin, eye, or respiratory irritation. Spilling the powder on a hot day always reminded me to double-check that my gloves and mask fit snugly. Materials Safety Data Sheets urge standard precautions—eye protection, gloves, fume hoods, and avoidance of dust inhalation. Good practice includes spill kits on hand and labeling containers, making auditors happy and keeping lab techs healthy. Regulatory benchmarks like REACH and OSHA standards guide permissible exposure levels and waste handling. Disposal involves neutralization and washing through properly filtered systems. Emergency procedures rarely get used, but drills and safety signage mean everyone reacts quickly if a bulk drum leaks or a scale pan overflows.
Water treatment, dye synthesis, and phase transfer catalysis stand out in industry usage. In manufacturing dyes, the m-xylenesulfonic acid sodium salt acts as a stabilizer or dispersing agent, making pigment mixing and textile dyeing more precise and robust. Water-soluble sulfonic salts often help soften water by suppressing scale-forming ions due to strong ionic interactions. I’ve seen this chemical in pilot plants and full-scale reactors, enabling modular mixing of aqueous solutions that would otherwise risk caking or incomplete dissolution. Many researchers studying custom surfactants or catalysts lean on the sodium salt as a testbed for how sulfonic groups change solubility and functionality. Even municipal water frameworks use it or its kin to regulate mineral content and reduce clogging, extending the life of pipes and pumps.
Toxicity studies so far find acute risk limited, though chronic exposure data remains underexplored. Studies using fish and plant models reveal low-to-moderate ecotoxicity; this makes sense given the salt’s high water solubility and tendency to disperse rather than bioaccumulate. Still, breakdown products and long-term soil contact need more scrutiny. I’ve worked with environmental chemists running degradation studies, revealing that sunlight and microbial action can, over some weeks or months, diminish residual concentrations in effluent streams. Product stewardship in the chemical industry depends on sharing new ecotoxicology data openly. Labs conducting risk assessments focus on real-world exposure: drum handling, wastewater treatment, fire scenarios. Regulatory filings now demand ongoing updates as new studies turn up, so companies who stay ahead with rigorous in-house testing fare much better during audits and certifications.
Product innovation comes from pressure: demand for greener chemistry, changes in regulatory thresholds, or the call for high-performing components in electronics and pharmaceuticals. Newer synthetic approaches look at bio-based routes that move away from fossil starting materials. I’ve seen startups prototype enzymatic sulfonation processes, aiming for higher selectivity and cleaner profiles. The push for more biodegradable auxiliary chemicals in detergent and dye industries keeps labs searching for analogues that deliver comparable function with a better environmental footprint. Machine learning shapes future research, as screening databases predict where modified sulfonates outperform legacy products. Companies looking for breakthroughs collaborate with universities, testing modifications for everything from conductivity in polymer electrolytes to antimicrobial coatings.
Crafting and using m-xylenesulfonic acid sodium salt reflects the evolution of applied chemistry: curiosity turned into practical tools. Keeping an eye on chemical safety, transparency in labeling, and a readiness to embrace more sustainable options will set apart manufacturers and researchers who want to lead, not follow. The story of this compound keeps unfolding, driven by those willing to test new ideas and refine the details that make large-scale application possible. By inviting feedback across fields—environment, manufacturing, medicine—the base knowledge on m-xylenesulfonic acid sodium salt keeps growing, pushing boundaries in creative and sometimes unexpected ways.
Chemicals like M-Xylenesulfonic Acid Sodium Salt rarely show up on mainstream news, but they shape a lot of what we use every day. I remember visiting an industrial plant some years ago, staring at drums whose labels only a lab chemist could untangle. Turns out, these compounds do more than most of us realize, especially in cleaning, dyeing, and even drug manufacturing.
Ask anyone working in textiles or pigments: M-Xylenesulfonic Acid Sodium Salt helps dyes dissolve better and spread evenly. Water and oil don’t like each other, so dye factories use chemicals that bridge the gap. This salt acts as a surfactant; it lets water mingle with stuff that’d normally bead up and roll off. Anyone who’s spilled oil on their shirt and struggled to clean it knows the power of surfactants. Without them, factory batches would turn streaky or clumpy, and the color would set unevenly. Fewer ruined batches means less waste and lower costs—a win for business and the planet.
Places that churn out car parts or electronics often fight an endless battle against oily grime. The chemicals in strong cleaners rely on sodium salts like this one. It doesn’t just power clean floors or greasy hands; it stops residue from sticking back to clean surfaces. I’ve seen its effect on factory floors: less elbow grease and faster routines, with safer results because workers don’t spend hours exposed to harsh chemicals. Compared to older, harsher acids and bases, these salts offer a friendlier way to break down industrial messes, especially in places with strict health rules.
Chemists don’t spend years searching for new drugs without plenty of backups. In labs, this sodium salt speeds up reactions without becoming part of the final medicine. By working as a catalyst, it keeps the process flowing, which allows for more precise control over the end product. That’s not just a technical benefit. A well-controlled reaction means a drug that meets strict safety tests, every single time. Skipping or switching the catalyst can mean thousands of dollars lost on failed attempts.
No specialty chemical comes without risk, and folks who work with M-Xylenesulfonic Acid Sodium Salt have to handle it carefully. Sometimes, factories release leftovers into water or air, which brings up big questions about long-term pollution. Regulations in the US, Europe, and parts of Asia push companies to keep spills and emissions to a minimum. I’ve spoken with water treatment engineers who constantly monitor for sulfonic acids because small leaks can harm fish and plants. Luckily, some new methods recycle or neutralize these salts before disposal, so we’re not stuck with yesterday’s mistakes.
Based on facts from groups like the EPA and industry watchdogs, those handling M-Xylenesulfonic Acid Sodium Salt need solid training and equipment. Companies investing in scrubbers and containment save money and trouble in the long run. Manufacturers can swap to greener substitutes in some cases, cutting hazards at the source. Real progress comes when labs, factories, and regulators share practical data and better techniques out loud, not just among experts. That’s how safer chemistry keeps pace with demand outside our lab doors.
Walking through the world of chemicals can be confusing, especially when complex names pop up, like M-Xylenesulfonic Acid Sodium Salt. Without a clear formula, both scientists and those working with chemicals outside the lab face uncertainty. The real issue comes from inconsistency between product labels, data sheets, and even academic sources. M-Xylenesulfonic Acid Sodium Salt brings together two main pieces: the aromatic ring from xylene and the sulfonic acid group, with the sodium ion added in. By naming the structure directly, the chemical formula for M-Xylenesulfonic Acid Sodium Salt is C8H9SO3Na.
From my own chemistry background, figuring out these formulas often meant sitting at a lab desk, searching through textbooks and double-checking aromatic ring substitutions. Let’s walk through it: m-xylene has two methyl groups on a benzene ring, sitting at the 1- and 3-positions. Add a sulfonic acid group in, usually at the 4-position for meta orientation. Then comes the sodium salt part. The hydrogen from the -SO3H gets swapped out for a sodium ion, turning it into a stable salt.
So, breaking it into atoms, the formula lines up as follows:
Keeping chemical formulas straight impacts more than just lessons in school. Accurate identification makes the difference in storage guidelines, safety protocols, and transport. The sodium salt form of m-xylenesulfonic acid tends to show up as a white crystalline powder, dissolves well in water, and acts as a common intermediate for dyes and other chemicals. If someone confuses it with a different isomer or gets the formula mixed up, accidents can happen. Even the wrong data in a safety sheet might send someone searching for the wrong antidote if spills occur. In my early days as a lab assistant, I saw how just a single typo in a formula could send an entire batch into waste.
Access to correct molecular formulas like C8H9SO3Na allows industry workers, researchers, and teachers to focus less on basic corrections and more on solving real problems. Sharing accurate information keeps research honest and stops companies from making costly mistakes. If you’re collecting materials for research or production, checking formulas through verified databases (like the PubChem or ChemSpider entries) prevents confusion and limits the risk of dangerous mixups. I always double-check new suppliers with trusted chemical registries before any order leaves the storage shelf.
All this comes down to building a habit of verification. In science, trust relies on clarity—names and formulas form the base of so many choices, from classroom lessons to process engineering. For M-Xylenesulfonic Acid Sodium Salt, C8H9SO3Na stands out as the precise answer, helping everyone avoid setbacks and keep their workflow a little smoother.
People toss around a lot of talk about chemicals and their impact on health. M-Xylenesulfonic Acid Sodium Salt pops up in industrial settings, research labs, even in specialty cleaning blends. Some encounter the name and wonder: are there real dangers hiding behind this dry, unassuming name?
I’ve poked through safety datasheets more times than I can count. The story for this particular salt isn’t the tale of a hidden monster, but it isn’t as harmless as table sugar. Many safety datasheets flag the powder for causing skin or eye irritation. I brushed a sleeve against a similar sulfonic salt once without gloves, and the itching flared up fast. The concern grows if fine dust is inhaled. Nobody wants that kind of persistent cough, especially lab workers spending hours inside. Chronic exposure carries more risk for those who aren’t careful about ventilation or personal protective equipment.
Now, looking at toxicological data, things get clearer. Acute oral toxicity in rats lands at fairly high doses, not right off the bat. It doesn’t match the danger level of cyanide or mercury, nowhere close. But “not acutely toxic” doesn’t mean you can toss safety out the window. Even substances considered “low hazard” can create problems over time, especially for people with sensitive skin or underlying breathing troubles.
Many chemicals sit in a gray area because studies never cover every scenario. M-Xylenesulfonic Acid Sodium Salt has limited long-term health data in public research. Without clear evidence, some users fall back on gut feel. Science favors caution, especially for substances used around open skin or in the air. The Environmental Protection Agency and similar agencies haven’t stamped strong warning labels on this salt, but they do ask for basic precautions. Gloves, goggles, and decent room ventilation go a long way in keeping exposure in line.
Supporting EvidenceAccording to the European Chemicals Agency, this compound gets classified as an irritant but not as acutely toxic. Those facts line up with first-hand accounts from both process engineers and bench scientists. I remember a former colleague who handled it in college chemistry, and the warning labels made us keep it off our hands and never taste or sniff it up close. In that sense, common sense lined up with regulatory advice. No severe cases hit the news or medical reviews, but you won’t find anyone in the field treating it like flour or sugar—the respect is there, even if acute harm is rare.
Many people forget how quickly an irritation can ruin a workday—and add up over time. All the best practices around chemical safety—wearing the right gloves, using splash goggles, running good airflow—help prevent almost all known problems with substances like this. For schools and businesses, investing in basic training and posting up-to-date safety sheets on doors and shelves isn’t an outdated tradition. The safer habits grow from moments like kids learning the difference between “irritant” and “toxic,” practicing safe handling so everyone goes home healthy.
Ultimately, there’s no headline scare from M-Xylenesulfonic Acid Sodium Salt. Like many specialized chemicals, the story is about respect, routine checks, and knowing a gloves-on approach still matters—even for compounds lingering in science’s quieter corners.
Storing chemicals in a safe and sensible way means looking at more than a label. M-Xylenesulfonic Acid Sodium Salt isn’t something that gets tossed alongside office supplies. My first real warehouse job hammered it in: safety is built step by step, not by shortcuts. Even if the name sounds technical, what matters is respecting its properties and the risks tied to salt-based acids. A careless approach can bring big trouble, not just for workers but for the environment and anyone downstream from a spill or mistake.
This salt often travels as a powder or crystalline solid. Exposure to high humidity or moisture can clump it up or start a reaction you didn’t plan for. It makes sense to pick a dry spot, away from the mess of pipes or leaks. Temperatures should stay stable, away from furnace rooms or sun-blasted windows. Some folks forget how direct heat can mess up chemical integrity, possibly causing the substance to break down or change. Cold isn’t as big an enemy for this compound, but wild swings ruin consistency.
I learned early to keep chemicals with similar risks in dedicated areas. Throwing everything together puts people and stocks in jeopardy. As an acid salt, it reacts poorly with oxidizers, strong acids, and bases. Even accidental mix-ups can lead to dangerous fumes or fires. That risk multiplies when storage drums or bags rip and contents mingle on a dusty warehouse floor. Color coding on shelves or firm access rules stop these messes before they start.
Experience tells me never to trust brittle or worn containers. Chemicals like M-Xylenesulfonic Acid Sodium Salt need airtight, non-reactive packaging—HDPE or sturdy drums outlast cheap plastic bags every time. Strong seals help avoid the headaches of spills, powder leaks, or accidental cross-contact. Labels should shout content clearly, using industry standards, with handling instructions in plain view. Anyone working late or moving quickly must see danger at a glance, not hunt through paperwork. A sharp label saves more panic and injuries than any thick rulebook.
Some chemical dusts cause sneezing, coughing, or worse. Anyone moving this material should slip on gloves, goggles, and masks—not as an act, but because one misjudged scoop means hours of eye-watering misery or a trip to the nurse. Local exhaust ventilation isn’t overkill for transfers or repacking; it keeps lingering dust from causing rashes or accidental ingestion. I remember sweating in a mask during a long shift, but learned even cheap gear blocks powder clouds and cuts complaints at the end of the week.
Chemical residues don’t just disappear. Sweeping spills into the trash or washing them down the drain only kicks the problem to someone else. Simple kits—neutralizers, absorbent pads, and strong bags—avoid bigger headaches. Clear emergency protocol beats improvising under pressure. From what I’ve seen, a few minutes spent planning makes all the difference between a minor incident and a shutdown. Every bag or drum of M-Xylenesulfonic Acid Sodium Salt deserves a safe route from shelf to use, then on to responsible disposal.
In the big picture, training workers and supervisors pays off more than fancy security systems. A solid short course on hazards, gear, and routine checks goes further than any amount of written policy kept in a binder. My teams always did better after walking through their own storage areas, pointing out which habits to drop or keep. Respect for chemical rights of way and routine checks became second nature, not just another safety poster on the wall.
Having spent years working alongside chemists and teachers in labs both dusty and high-tech, I’ve learned that not all chemicals dissolve in water with the same ease. Some compounds surprise you—they disappear quickly, turning a clear solution that seems to erase all signs of the starting powder. M-Xylenesulfonic acid sodium salt falls into that family. This substance doesn’t hang around in clumps on the bottom of your beaker. It enters solution rapidly and fully, showing one of the key properties that lets it fit easily into both research labs and industry alike.
M-Xylenesulfonic acid sodium salt boasts high solubility in water. Experienced lab techs will tell you that a standard solution of this salt dissolves without need for vigorous stirring or extra heat. At room temperature, you can expect more than 200 grams of this salt to dissolve in a single liter of water. That’s considerably higher than sodium chloride (table salt), and it’s this high solubility that makes the compound so appealing in real-world work.
The practical implications show up pretty quickly. The salt will help scientists prep solutions for experiments in analytical chemistry, electrochemistry, or dye manufacture. You don’t lose precious time waiting for stubborn undissolved bits. In wastewater treatment, the solubility lets it serve as a strong source of sulfonate groups for ion exchange resins or help adjust pH in specialty processes. Myself, I’ve found the moments of quick, clean dissolution always help a day in the lab go smoother.
Legitimate sources back up these solubility claims. Trusted chemical suppliers and chemistry resources often list “freely soluble” or “very soluble” in their specifications. Literature on arylsulfonate salts confirms they tend to dissolve well in polar solvents like water. In practice, you’ll see this trait used in applications where uniform distribution or rapid reaction is necessary. Having reliable data means researchers and process engineers can plan recipes with confidence, knowing the salt will dissolve as needed.
Good solubility isn’t a pure blessing. High levels can sometimes make it trickier to recover the salt from solution if you need it back. This means more steps and more energy for recovery or purification. Also, if waste streams from manufacturing run into city wastewater systems, easy dissociation in water could pose environmental questions. Experienced hands treat strong sulfonate salts with respect in disposal, following established safety and environmental protocols.
Those working with chemicals like m-xylenesulfonic acid sodium salt shoulder a responsibility. High solubility brings both convenience and a challenge in handling discharge. Laboratories and factories need robust procedures for containment and neutralization. Wastewater treatment plants should keep an eye out for unusual levels. Identifying opportunities for recycling or closed-loop systems helps limit impact. Many facilities now audit their chemical inventories and revisit disposal methods, sometimes pairing chemistry with new filtration or advanced oxidation systems, to do right by both science and the environment.
Working alongside experienced professionals, I’ve learned that respecting the solubility of compounds in water allows for both safer work and cleaner results. M-Xylenesulfonic acid sodium salt’s ability to quickly integrate into aqueous solutions opens doors, but practical responsibility still matters.
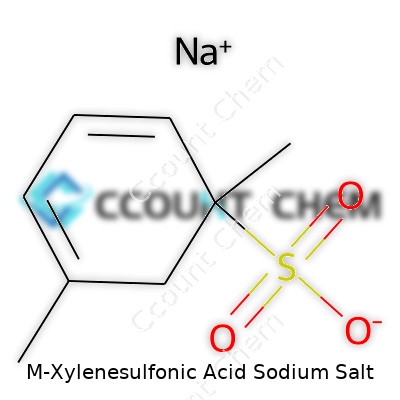
| Names | |
| Preferred IUPAC name | Sodium 3-methylbenzenesulfonate |
| Other names |
Sodium 3-methylbenzenesulfonate m-Xylene-3-sulfonic acid sodium salt Sodium m-xylenesulfonate |
| Pronunciation | /ɛm-zaɪˌliːn.sʌlˈfɒn.ɪk ˈæs.ɪd ˈsoʊ.di.əm sɒlt/ |
| Identifiers | |
| CAS Number | 1300-72-7 |
| 3D model (JSmol) | `3D structure; InChI=1S/C8H8O3S.Na/c1-6-3-4-7(2)8(5-6)12(9,10)11;/h3-5H,1-2H3,(H,9,10,11);/q;+1/p-1` |
| Beilstein Reference | 1309288 |
| ChEBI | CHEBI:91214 |
| ChEMBL | CHEMBL1906715 |
| ChemSpider | 23457 |
| DrugBank | DB14466 |
| ECHA InfoCard | 100.027.666 |
| EC Number | 216-104-4 |
| Gmelin Reference | 113209 |
| KEGG | C18713 |
| MeSH | D014527 |
| PubChem CID | 23674757 |
| RTECS number | DO2275000 |
| UNII | 7E8Z89793Y |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID40985879 |
| Properties | |
| Chemical formula | C8H9NaO3S |
| Molar mass | 196.20 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.31 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.0 |
| Vapor pressure | <0.01 mm Hg (20°C) |
| Acidity (pKa) | pKa ≈ -2.0 |
| Basicity (pKb) | 13.1 |
| Magnetic susceptibility (χ) | -57.2·10^-6 cm³/mol |
| Refractive index (nD) | 1.523 |
| Viscosity | 30 mPa·s (20°C) |
| Dipole moment | 4.28 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 240.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -663.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3946.8 kJ/mol |
| Pharmacology | |
| ATC code | R05CB19 |
| Hazards | |
| Main hazards | Irritating to eyes, skin and respiratory system. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-0-1 |
| Flash point | > 181°C |
| Lethal dose or concentration | LD50 Oral Rat 2480 mg/kg |
| LD50 (median dose) | LD50 (median dose) of M-Xylenesulfonic Acid Sodium Salt: "LD50 oral (rat) > 2000 mg/kg |
| NIOSH | WX8575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
m-Xylenesulfonic acid o-Xylenesulfonic acid sodium salt p-Xylenesulfonic acid sodium salt Toluene-4-sulfonic acid sodium salt Benzene sulfonic acid sodium salt |