Chemistry follows trends just like any other field, and the story of m-xylenesulfonic acid traces decades of work devoted to understanding aromatic sulfonation. Back in the industrial heyday, researchers searched for ways to improve dye intermediates and detergents, leading them right to sulfonic acid derivatives. Aromatic sulfonation became a staple in organic synthesis. The discovery of m-xylene’s reactivity gave researchers an easy entry point. Sulfonating m-xylene provided a sturdy platform for modifications, leading researchers during the twentieth century to fine-tune reaction conditions and production scale. Once pharmaceutical, photographic, and surfactant fields picked up speed in the 1960s and 1970s, the demand for this acid motivated large-scale adoption, especially in Asia and Europe, continuing into today’s specialty chemical industry.
M-xylenesulfonic acid looks fairly unremarkable—a crystalline solid or sometimes a syrupy liquid, usually colorless or pale yellow, and often packed in sturdy drums. It’s not as showy as some specialty chemicals but plays a part behind the scenes in dye manufacture, surfactant synthesis, and even pharmaceutical intermediates. Production typically follows orders in bulk for industry clients; it rarely grabs shelf space in academic labs, although anyone who synthesizes with aromatic systems eventually hears its name. The product’s use depends on both its reactivity and its compatibility with a range of organic molecules, giving it a flexibility valued by synthetic chemists and industrial process engineers alike.
At room temperature, m-xylenesulfonic acid stands out for its solid state and relatively high melting point compared to its parent hydrocarbon, m-xylene. This chemical dissolves with some vigor in water, aided by its sulfonic acid group, which gives it strong acidity. Even at modest concentrations, the acid’s solutions turn litmus deep red and react strongly with many organic and inorganic bases. That acidity doesn’t fade easily—the sulfonic group brings stability against hydrolysis and oxidation, so the substance keeps its punch even with heat or mild oxidizers. This stability fuels its use in processes demanding harsh conditions, such as sulfonation or dehydration reactions.
Quality control isn’t just about checking purity anymore—it can make or break downstream processes. Industrial suppliers typically confirm assay levels of the main compound, often above 98%, while controlling for water content, residual sulfur dioxide, and heavy metal traces. Labels follow both local and international regulations, listing the chemical’s hazard warnings, proper handling recommendations, and any critical registration data such as CAS numbers or EC numbers. In logistics, packaging uses acid-resistant lining and tight sealing to keep out moisture and prevent leaks. Standard practice cites m-xylenesulfonic acid’s classification as both an irritant and a corrosive, requiring clear markings so handlers know what they’re working with.
Synthesis of m-xylenesulfonic acid relies on the classic sulfonation route. Manufacturers treat m-xylene with concentrated sulfuric acid or oleum, usually under controlled temperature and agitation. M-xylene’s structure brings the sulfonic group onto the meta position, thanks to the steric effects of the two methyl groups. After neutralization, the acid form can be isolated, often through crystallization or extraction, with downstream steps to dry and refine the product. Large producers may use continuous-flow reactors or batch processing, depending on output volume and cost concerns.
In the hands of an organic chemist, m-xylenesulfonic acid takes on several roles. It works as a strong acid catalyst in esterification or alkylation. Reactive sulfonic acid groups enable further transformations, making the substance a reliable intermediate for sulfonamide formation, for coupling reactions in dye synthesis, and for deactivation steps in aromatic substitutions. Under reduction conditions, the sulfonic group can be selectively removed or replaced with other functional groups, expanding its utility in multi-step syntheses. For surfactant chemists, reacting this acid with bases such as sodium hydroxide produces the corresponding m-xylenesulfonate salt, a classic detergent additive.
Over the years, this compound has picked up quite a list of synonyms. Names in literature and on product labels include 3-xylenesulfonic acid, meta-xylenesulfonic acid, m-xylene-3-sulfonic acid, and even some historic trade names used in dye and surfactant specification lists. Chemists often refer by CAS number for clarity in procurement or regulatory paperwork. Different manufacturers might market the compound using branded names when targeting niche sectors such as pigment intermediates, but most stick to clear chemical nomenclature for industry clarity.
Working with strong acids always reminded me of the day a careless pour etched a hole right through a glove—nobody wants a repeat of that drama. M-xylenesulfonic acid brings both strong acidity and some corrosive danger. Proper storage in acid-resistant containers, chemical goggles, and acid-proof gloves stand as baseline requirements for industrial and research settings. Facilities storing or using the substance keep spill kits and eye-wash facilities handy. Ventilation remains essential, especially in confined spaces. The relevant regulatory systems call for clear labeling, safety data sheets, and proper waste disposal to prevent both workplace injury and environmental release. Worker training—covering exposure response, neutralization procedures, and evacuation routes—comes as part of every induction involving this acid.
Every batch leaving the factory winds up playing a role in larger chemical chains. In my own time working around dye intermediates, m-xylenesulfonic acid cropped up regularly in the sulfonation step for azo dyes—the high reactivity gives crisp separation and vivid color yields. Pharmaceutical manufacturers often rely on its intermediates for antimicrobials and sulfa drugs. Outside these, the detergent industry processes the acid into its sodium salt, then blends it into formulations aimed at performance under hard-water conditions. Some resin synthesis protocols exploit its acid strength, especially during condensation reactions that demand punchy acid catalysis without exotic reaction waste. Even water treatment facilities draw on sulfonated aromatics for specialty resins and exchange media.
Scientists never quite stop tinkering with aromatic acids. R&D teams continue exploring milder sulfonation methods, aiming to reduce waste, increase selectivity, or lower overall carbon footprint. Green chemistry ideas focus on finding less hazardous sulfonation reagents or more recyclable solvents. Researchers probing alternative uses continue to look for catalytic opportunities or novel drug scaffolds. Process engineers investigate higher-yield syntheses using continuous-flow microreactors that promise improved control, safety, and scalability. The emergence of computer-aided synthetic planning aids researchers looking for new transformations and modifications, harnessing m-xylenesulfonic acid as a building block for new classes of materials and medicines.
Toxicology studies show m-xylenesulfonic acid sitting between common mineral acids and more exotic organics. Handling can cause burns, skin and eye irritation, and—if accidentally ingested or inhaled—serious mucous membrane damage. Published studies highlight the substance’s corrosive nature, but chronic toxicity appears limited if exposure remains low and well-controlled. Ecotoxicology papers suggest that the acid degrades moderately fast in surface waters, but sulfonated aromatics as a class sometimes persist and accumulate in soil or aquatic environments. Routine worker monitoring and ongoing research on its breakdown products help industry minimize impact both on the factory floor and in downstream waste streams.
Looking ahead, demand stays steady in traditional dye, detergent, and pharmaceutical sectors. Industry trends point toward greener production methods, stricter safety protocols, and value-added applications in high-performance materials. Regulations on aromatic sulfonates are tightening in the EU and parts of Asia, prompting companies to invest in cleaner processes and improved waste management. Meanwhile, academic and industrial labs keep looking for new ways to harness the versatile reactivity of m-xylenesulfonic acid, whether through biocatalysis, new surfactant technology, or as an intermediate in specialty polymers. There’s ongoing potential for material innovation, even as the core applications remain familiar to anyone working in synthetic organic chemistry or applied materials science.
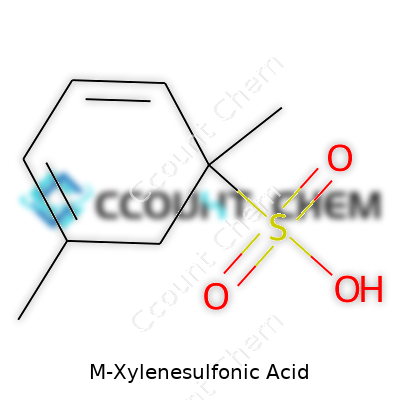
M-xylene sulfonic acid comes with the chemical formula C8H10O3S. That name may sound technical, but at its core, it describes a compound where m-xylene—a simple aromatic hydrocarbon—has a sulfonic acid group (-SO3H) attached to its ring. In my chemistry days, sometimes it felt like every new formula was a secret code, but these codes matter. They tell us about how molecules interact, behave, and influence daily life, from detergents to dyes, and much more.
Listing the formula C8H10O3S isn’t just an exercise in memorization. In every laboratory I’ve worked, knowing the exact structure helped us predict how the substance would react with everything from water to metals. Sulfonic acid groups bump up the acidity and solubility of these compounds. Manufacturers and researchers rely on this property. Take the world of cleaning agents: sulfonic acids show up often as starting materials, helping create soaps that can break through oil, dirt, and even ink stains.
Outside laboratory walls, m-xylenesulfonic acid and its relatives show up in dyes and pigments. My old chemistry professor used to say, “Color doesn’t stick around on fabric without the right chemical handshake”—and those handshakes often involve sulfonic acids. They help dye molecules grab onto fabric fibers and stay there, even after multiple washes. If you’ve ever worn a cotton T-shirt with a bold print, there’s a decent chance you’ve benefited from the chemistry behind it.
But every powerful chemical comes with downsides. Handling compounds like m-xylenesulfonic acid in the workplace can mean risks, especially if workers don’t have the right gear or training. Coming in contact with strong acids, even accidentally, can damage the skin and eyes fast. It’s crucial for anyone working with these chemicals to respect safety protocols. Personal experience in the lab showed that one small mistake—like not wearing goggles—can end a career or much worse.
From a broader perspective, discharge of sulfonic acids into streams and groundwater can upset aquatic life and harm communities downstream. Good waste management starts with real accountability at the site where the chemical is made or used. Companies that treat wastewater before it leaves their facilities already make a difference. Stricter enforcement of chemical safety rules keeps toxins out of water supplies, and worker training ensures that people handling these substances know what’s at stake.
Chemistry shapes our lives in ways most folks don’t notice, but these details matter. Knowing the formula for m-xylenesulfonic acid isn’t just a fact to recite, but a step toward better decisions in industry, health, and the environment. Digging into the structure, spotting the risks, and holding manufacturers and users responsible all add up to progress—one compound, one formula, one conscious choice at a time.
M-Xylenesulfonic acid lands in that group of specialty chemicals most people never hear about, yet it quietly supports a range of industries. If you’ve ever wondered what keeps dyehouses, chemical manufacturers, and even some water treatment facilities running smoothly, it’s not always the headline-making compounds—sometimes it’s practical workhorses like this one.
Let’s start with coloring. Textile mills and pigment manufacturers often use M-xylenesulfonic acid as an intermediate for developing dye precursors. It helps with sulfonation, which ultimately gives colorants their water solubility. The experience of seeing a vivid shirt hold its color after several washes? The roots trace back to acids like m-xylenesulfonic, which strengthen those dyes and provide them with staying power on fabrics. According to reports from the textile industry, sulfonic acids keep manufacturing costs in check while delivering the shades designers ask for season after season.
In organic chemistry, reactions need a push. M-xylenesulfonic acid shows up as a catalyst in several key reactions, from alkylation to esterification. A good catalyst saves time, improves yield, and helps cut down on unwanted byproducts. Companies in the resin manufacturing world often turn to this acid for its ability to get things moving without bringing on a tangle of side reactions. Having worked in a research lab, I’ve seen how a reliable catalyst can make or break a process. Minor efficiencies here save barrels of wasted material later.
Household and industrial cleaners owe plenty to the chemistry of sulfonic acids. M-xylenesulfonic acid gives detergent makers the backbone for making surfactants that wash away grime without holding back foam or effectiveness. The World Surfactant Association noted in a 2022 review how small tweaks in the production chain—swapping out a weaker acid for a sulfonic variant—slashed production costs by up to 15% while reducing chemical waste. This speaks volumes about the real impact these building blocks create in everyday cleaning products.
The pharmaceutical industry always looks for efficient ways to build complex molecules. M-xylenesulfonic acid steps in where selective sulfonation or controlled acidity is needed, guiding reactions to deliver cleaner yields. Antibiotic and antihistamine development both use the acid at important stages. Back in graduate school, I joined a synthesis project that relied on this acid—switching to it shaved weeks off our timeline, letting us finish optimization faster and get to animal trials sooner.
Handling acids in industrial settings always raises questions about safety and waste. M-xylenesulfonic acid’s relatively mild profile means it doesn’t require extreme handling. Still, it makes sense for companies to invest in closed-system handling, good ventilation, and worker safety training. Regulations in Europe and North America now push for greener disposal of sulfonic byproducts, driving manufacturers to adopt recycling technologies. In my time consulting for a mid-sized chemical plant, waste reduction strategies centered around acid reclamation cut disposal fees and protected local waterways—clear proof that both bottom lines and communities benefit from cleaner chemistry.
From improving lasting color in fabrics to making stronger detergents and medicines, M-xylenesulfonic acid shows how specialty chemicals quietly lift entire industries. As industries push for safer and cleaner operations, simple changes like better handling and waste recovery offer practical paths forward without sacrificing performance.
M-Xylenesulfonic acid isn’t your everyday chemical. Years spent around labs and warehouses have taught me to stay on my toes with these sorts of compounds. Known for its role in organic synthesis and as an intermediate in dyes and surfactants, this compound comes with a punch when it comes to safety. It’s a strong acid, corrosive, and can definitely make a mess of things if not handled with respect.
One of the key lessons I’ve learned—never cut corners with storage. M-Xylenesulfonic acid stores best in tightly sealed containers, made of materials that don’t react with acids. Glass and certain plastics, like polyethylene or Teflon, hold up well. Metal won’t, and that’s from personal observation after seeing what even a bit of acid can do to an unlined steel shelf. Leaks or cracks can lead to dangerous spills, so containers always deserve a good look. If a drum looks worn or if the cap isn’t clicking shut, swap it before you regret it.
Temperature also matters. This chemical gives off fumes that bite at the nose and eyes, especially in warm conditions. Keep storage cool, dry, and shaded. Direct sunlight or heat sources only invite trouble by raising the vapor pressure or even causing the acid to degrade. For me, a dedicated, ventilated room always wins out. A simple exhaust fan or proper hood goes a long way in keeping vapors from building up—something you’ll appreciate the first time you crack open a container on a humid day.
You never want to go hands-on with m-xylenesulfonic acid without the right gear. A lab coat, chemical splash goggles, and acid-resistant gloves have kept my skin safe many times. I always keep a face shield within reach when pouring or transferring. Even a small splash will sting and may burn clothing, skin, or eyes in seconds. Spills on the floor make for slick, dangerous surfaces that are no fun to mop up, so a solid pair of boots makes sense. Respiratory protection isn’t overkill either, especially if there’s any chance of inhaling vapors in a tight space.
Anyone who stores m-xylenesulfonic acid should think ahead about accidents. Neutralizing spills with sodium bicarbonate or lime helps control the acid before mopping up. Absorbent pads suited for acids trap small puddles. I always check that emergency showers and eyewash stations work, since quick action can make all the difference. Chemical spill kits should stay close, not locked in the next building over.
Good inventory records matter too. Know how much you’re storing, check expiration dates, and keep incompatible chemicals—like oxidizers or strong bases—elsewhere. Clear labels and up-to-date Safety Data Sheets make sure everyone’s on the same page. In my experience, a well-trained crew with regular drills stays safer and reacts faster when seconds count.
Proper handling of m-xylenesulfonic acid boils down to respect and routine. With regular inspections, careful attention to how chemicals are stored and handled, and making safety equipment part of daily work, the risks go down. Training helps everyone keep hazards top of mind. Keeping pace with best practices, backed up by solid, hands-on experience, can mean the difference between a routine day and a rushed trip to the emergency room. Safety becomes a habit—and that’s what keeps people going home at the end of the day.
People working with chemicals quickly learn that not every liquid in a beaker deserves the same respect as table salt. M-Xylenesulfonic acid carries a sharp reminder of that lesson. This compound, made from m-xylene and sulfonation agents, finds its way into dyes, fabric treatments, and catalysts. At first glance, it seems like just another industrial acid. Step closer, and you’ll see why real caution is called for.
Acids have a reputation, and this one earns it. Skin contact with m-xylenesulfonic acid results in burning, peeling, and even long-lasting tissue damage. Eyes take the brunt—redness, swelling, risk of permanent vision loss if splashed without protection. Vapor and mist, not just liquid, put lungs at risk. Breathing it in triggers coughing, sore throats, and sometimes serious inflammation deep in the chest. It’s not just theory either; workplace records make it clear that accidental splashes and spills bring people to emergency rooms every year.
Chemicals don’t stay put. Spilled m-xylenesulfonic acid seeps into drains, where it harms fish, aquatic plants, and even bugs that keep ecosystems balanced. Acidic runoff affects pH, and some breakdown products disrupt local wildlife. In industrial zones near streams or ditches, even a small release travels far. Safety isn't only about the people handling the acid—it’s about the places that rely on clean water and uncontaminated soil.
I spent summers in a manufacturing plant storing dozens of acid drums in racks that looked as sturdy as highways. Nobody felt casual about m-xylenesulfonic acid. Management posted goggles and face shields right at the entrances. I saw a veteran worker, gloves forgotten, wipe his brow absentmindedly and land in the nurse’s office with burns within minutes. That incident reshaped how all of us treated lab routines—gloves, aprons, and respirators went from ‘good practice’ to jobs on the line if ignored. You never forget the sight of that kind of chemical injury.
Best practice begins before a drop comes out of a drum. Only trained staff should handle the acid. Full chemical-resistant gloves make a real difference—nitrile or neoprene outlast regular latex every time. Goggles are essential, but face shields add another layer. Lab coats stop splashes from eating into clothes and skin. If any risk of vapor exists, or the job involves pouring into open containers, a proper respirator keeps airways safe. Workers need clear, simple steps on what to do during spills: acid-neutralizing agents on standby, spill kits at arm’s reach, and emergency eyewash stations nearby.
Storage matters too. Keep containers tightly sealed, stored away from bases or oxidizers. Every plant worth its insurance keeps acids in separate, well-ventilated rooms. Never reuse old food or drink containers. It only takes one wrong label or faded sticker to cause real confusion and danger.
Policy matters just as much as equipment. Strong managers give space for people to report close calls without punishment. New hires see veteran employees follow the same safety steps every day—habits form faster than rules stick. Rewards for safe handling don’t cost much and signal that management expects prevention, not damage control.
M-xylene sulfonic acid offers real industrial value but comes with real risk. Treating each shift in the lab or plant like a chance to stay sharp, not cut corners, builds safety for everyone—at work and downstream in the environment. That mindset doesn’t just keep plants running, it keeps people and their communities healthier too.
M-Xylenesulfonic acid isn’t as famous as sulfuric acid or nitric acid, but in certain labs and plants, it shows up often enough. I’ve seen folks get lazy about chemical disposal and pretend it all vanishes down the drain. That’s just a recipe for long-term trouble. Sulfonic acids, including this one, mix well with water. They slide into groundwater and end up in rivers and lakes where water treatment plants can’t always catch up. Fish and invertebrates in those ecosystems have no defense. I remember an old colleague mentioning local drinking water tests turning up traces of lab chemicals dumped decades ago. Fast moves with waste turn into headaches later.
OSHA and EPA rules step in for a reason. Tossing M-Xylenesulfonic acid in the regular trash or sink lands folks in legal trouble and leaves a mess for someone else. Handling these acids asks for sensible practices, not just following forms but real caution.
Neutralization stands out as one of the first tactics for minimizing the hazard. I always take a measured approach: adding a base like sodium carbonate or sodium hydroxide to the acid. The reaction should go slow—big splashes and heat belong in comic books, not labs or shops. Even once the acid is neutralized to a near-neutral pH, it’s not time to kick back. The resulting salts won’t carry the same bite, but they still can harm waterways if washed away in bulk. Holding the liquid for professional disposal, or sending it via an approved hazardous waste facility, makes more sense.
Large-scale users would be smart to collect spent acid in labeled, sealed containers. Ignore the temptation of old soda bottles or rusty paint cans. Leakproof, chemical-resistant jugs marked with the right labels keep peace with inspectors and avoid accidents. I’ve witnessed enough confusion over mystery containers to vouch for good tracking and clear labels—no one wants to be the person who finds out the hard way what’s inside.
Small shops or school labs face limits. Not everyone has budget or space for fancy treatment rigs, so a reliable hazardous waste hauler pays off. Building that relationship beats last-minute Google searches after something spills. There’s nothing glamorous about dragging bottles to the pickup point, but it beats polluting the backyard. In my own practice, logging every chemical transfer and having a regular disposal schedule sets a gold standard that others notice and copy.
On a bigger scale, industry can cut down on waste by reusing or recycling acids when possible. Some industrial outfits distill old acid for reuse or convert it into less hazardous byproducts, but that’s not in reach for everyone. At the very least, combining waste streams makes disaster—the wrong chemicals can react and produce toxic gases or worse. Mixing without thought has burned more than one careless tech.
Education beats after-the-fact fines. Training staff—students, lab workers, or factory floor crews—pays dividends. Walk them through what happens if that jug of m-xylenesulfonic acid just “disappears.” Real stories stick better than warning labels. The next generation of chemists and techs won’t protect the environment unless they’ve seen how small choices add up. In my eyes, habits learned with these substances echo for life.
In the end, everyone in the chain—producer, user, and disposer—shares the responsibility for proper treatment of M-Xylenesulfonic acid. A little extra effort saves a lot of future regret, and cleaner water for all.
| Names | |
| Preferred IUPAC name | 3-Methylbenzenesulfonic acid |
| Other names |
m-Xylene-4-sulfonic acid 3-Xylenesulfonic acid m-Xylene sulphonic acid |
| Pronunciation | /ɛm-zaɪˌliːniːsʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 7034-48-3 |
| 3D model (JSmol) | `CC1=CC=CC(=C1)S(=O)(=O)O` |
| Beilstein Reference | 1718763 |
| ChEBI | CHEBI:27552 |
| ChEMBL | CHEMBL504040 |
| ChemSpider | 186417 |
| DrugBank | DB04112 |
| ECHA InfoCard | 14e171f6-c9b6-4484-8b96-8a95baa969c7 |
| EC Number | 269-049-6 |
| Gmelin Reference | Gmelin Reference: **136223** |
| KEGG | C02322 |
| MeSH | D016849 |
| PubChem CID | 10815 |
| RTECS number | GO7350000 |
| UNII | M8G12L6I8F |
| UN number | UN2582 |
| CompTox Dashboard (EPA) | DTXSID1044288 |
| Properties | |
| Chemical formula | C8H10O3S |
| Molar mass | 170.21 g/mol |
| Appearance | Colorless to light yellow transparent liquid |
| Odor | Odorless |
| Density | 1.2 g/cm³ |
| Solubility in water | soluble |
| log P | -1.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | -2.5 |
| Basicity (pKb) | 12.52 |
| Magnetic susceptibility (χ) | -56.5 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.558 |
| Viscosity | 10 mPa·s (20 °C) |
| Dipole moment | 4.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -482.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3786.7 kJ/mol |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if swallowed, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | Precautionary statements for M-Xylenesulfonic Acid: "P264, P280, P301+P330+P331, P305+P351+P338, P309+P311 |
| NFPA 704 (fire diamond) | 3-1-2-Acidos |
| Flash point | 102 °C (216 °F; 375 K) |
| Autoignition temperature | Autoignition temperature: 550°C (1022°F) |
| Lethal dose or concentration | Lethal dose or concentration: LD50 (oral, rat): 1340 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2480 mg/kg |
| NIOSH | B0032 |
| PEL (Permissible) | Not established |
| REL (Recommended) | REL (Recommended Exposure Limit) of M-Xylenesulfonic Acid: "3 mg/m3 |
| IDLH (Immediate danger) | IDLH: 40 ppm |
| Related compounds | |
| Related compounds |
Benzene sulfonic acid p-Toluenesulfonic acid o-Xylenesulfonic acid m-Xylene m-Xylenol |