In the late 19th century, chemists carved new territory into organic chemistry with the discovery of m-cresol sulfonic acid. This compound comes from the family of cresols, derivatives of phenol, and caught researchers’ attention after advances in coal tar distillation. The need for stronger acids sent many industrial laboratories hunting for chemicals that could match and surpass what sulfuric acid alone could do. In time, manufacturers started introducing m-cresol sulfonic acid into processes that needed acidic catalysts with specific aromatic attributes. Uses in dye and resin synthesis led to larger commercial demand, and refining techniques saw significant improvements by the mid-20th century. Chemical companies responded by optimizing both the yield and purity of the compound, allowing it to become a go-to intermediate for a growing web of industrial applications.
M-cresol sulfonic acid arrives as a viscous, dark brown to amber liquid or solid, depending on temperature and concentration. Its aroma signals its phenolic roots, and the sharp smell sticks with anyone working nearby. Industrial supply usually involves pure or technical grades. Manufacturers and end-users who need consistent catalytic activity or reactive intermediates reach for this particular sulfonic acid, owing to its ability to combine a strong acid function with the reactivity of a methylated aromatic ring. In my own days inside a chemical plant, I saw this compound trigger reactions seamlessly in both batch and continuous production, especially when compared to simpler sulfonic acids.
The acid appears as a highly hygroscopic substance, eager to pull water out of any airspace it finds. Its solubility in water stands high, creating exothermic solutions due to its strong acidic nature. With a molecular weight around 172.18 g/mol, the solid form melts at roughly 105°C, though the exact value sways with purity and isomer content. The reactivity shoots up because both the sulfonic and hydroxyl groups love to donate or accept hydrogen ions in various conditions, giving the compound a resilience in both aqueous and organic phases. In the lab, I learned to respect its volatility and staining character, since a drop will leave a yellow-brown spot on skin, benchtop, and even steel.
Industry shipments arrive labeled with purity percentages, water content, and presence of other cresol isomers. Shipping regulations call for UN labeling due to its corrosive nature and toxicity. Companies typically pack the acid in lined drums or intermediate bulk containers, including material safety data and batch-specific documentation to make sure both buyers and handlers know the risks at every step. The need for traceability in large-scale pharmaceutical and resin production pushes firms to list origin, synthesis date, and analysis results. Over time, clearer labeling and thorough documentation have played an obvious role in preventing costly mistakes and potential exposures during transportation and storage.
The most common pathway for preparing m-cresol sulfonic acid starts with the direct sulfonation of m-cresol. This means adding fuming sulfuric acid or chlorosulfonic acid dropwise to a cooled batch of m-cresol under controlled conditions. The exothermic reaction produces the sulfonic acid, often alongside ortho- and para-isomers that later require separation. From my own participation in pilot plant scale-ups, maintaining low temperature and slow addition rates proved vital. Rushing any step here leads to side-products, lower yield, and equipment damage because of overheating or localized acid concentrations. Workers monitor color changes and heat output continuously, and post-reaction neutralization involves lots of ice and dilute alkali.
M-cresol sulfonic acid acts as a robust sulfonating and dehydrating agent. Its chemical activity enables alkylations, condensations, and polymerizations. Some resin chemists combine it with formaldehyde or other aldehydes to generate crosslinked phenolic resins. Sulfonic acid groups also boost water solubility in dye intermediates. Modifying the molecule through further alkylation or by neutralizing the acid group extends its utility into detergent and surfactant synthesis. Its methyl group resists most gentle oxidations, yet vigorous oxidants flip it to carboxylic acids. These modifications let chemists tune solubility and reactivity, depending on each application.
Industry circles and scientific literature feature several names for this acid, adding confusion for new researchers or purchasing agents. “Metacresol sulfonic acid” or “3-methylphenol-4-sulfonic acid” reflect the same core structure. In some catalogs, you’ll catch “MCSA,” “cresol-3-sulfonic acid,” or explicit CAS numbers. Suppliers outside the United States sometimes market the product under local language names or abbreviations. Internally, chemical companies use batch numbers and custom trade names that signal the acid’s grade, concentration, or isomeric purity, all to keep order in an industry with dozens of similar compounds.
Handling m-cresol sulfonic acid calls for respect. With its strong acid group and phenolic nature, it can burn skin, corrode metals, and damage plastics not rated for acid service. Respirators, acid-proof gloves, face shields, and heavy-duty aprons become daily wear for those working on loading docks, labs, or reactors. Spill kits stocked with neutralizing agents and absorbent pads stand ready in every hallway. Eye wash stations can save the day after accidental splashes. Workers get annual training on handling, storage, and first aid for cresolic compounds. On the regulatory side, agencies in the US and Europe demand regular reporting on workplace exposure, emissions and strict adherence to labeling and transportation codes.
Few compounds offer the versatility of m-cresol sulfonic acid across so many industries. In the dye world, it serves as a vital intermediate and catalyst, helping lock color onto fibers that might fade otherwise. Resin manufacturers turn to this acid to crank out thermosetting polymers used in electrical laminates and automotive components. Electroplaters and battery companies seek it out for its conductivity in electrolyte solutions. It shows up in oil refining as a phase transfer catalyst or additive, and in pharmaceuticals as a stepping stone in certain synthetic routes. Lab-scale organic synthesis leverages it as a dehydrating and activation reagent, particularly where milder acids fall short.
Research outfits keep expanding the boundaries of what you can do with m-cresol sulfonic acid. Recent years brought advances in green chemistry, where teams reworked old synthetic routes to limit hazardous byproducts and water use. Automated continuous-flow sulfonation systems cut down on operator exposure while boosting throughput. Improved catalyst recovery and regeneration methods help keep costs down in large batch operations. Suppliers also investigate new derivatives for use in specialty polymers and high-performance coatings. Scientists file new patents to cover applications in anti-corrosion treatments or as electrolyte additives for next-gen batteries.
Studies show that m-cresol sulfonic acid brings toxicity concerns both from its acid function and its cresol backbone. Human exposure at industrial concentrations leads to skin burns, respiratory damage, and potential systemic toxicity if splashed or inhaled. In animal tests and workplace monitoring, repeat exposure affected liver and kidney function, especially if personal protective equipment fell short. Wastewater discharge and unintentional spills pose risks to aquatic organisms, warranting strict effluent controls. Regulatory agencies in North America, Europe, and Asia update threshold limits and permissible exposure levels every few years based on ongoing epidemiological data and bench studies.
Demand for m-cresol sulfonic acid will not go away soon. As industries push toward greener chemistries, opportunities lie in process intensification, recovery, and recycling of acids used in catalysis. Improved control over purity and isomer content will let researchers fine-tune reactivity and performance, especially in high-value pharmaceuticals and performance polymers. Automated systems already cut the risk and manpower needed for batch processing, paving the way for new users and scaled-up operations in emerging markets. Efforts in toxicity reduction through inhibitor use and smarter waste management promise to keep the environmental footprint in check without hampering industrial innovation. In the coming decade, look for deeper integration into advanced materials and energy storage sectors as the world chases cleaner technologies.
Most people never cross paths with m-cresol sulfonic acid in daily life, but this substance shapes a surprising number of industrial processes. Industries rely on it to get things done faster, cleaner, or just plain better. Its chemistry reflects the know-how and problem-solving rooted in chemical manufacturing.
Anyone who's opened up a smartphone or computer knows how tightly packed those circuit boards get. Manufacturing them requires precision and clean surfaces. Companies use m-cresol sulfonic acid to clean and etch printed circuit boards. It dissolves residues and helps copper traces stand out crisp and reliable. High-quality electronics start with chemicals that don’t leave marks behind. Skipping this step means more defects and more waste—no one wants a phone that fizzles out after a month.
Dyes and pigments might sound a little old-school, but factories still count on acids like this to stitch color into fabrics, plastics, and inks. The molecule provides a strong sulfonating agent, letting dye manufacturers lock vivid color molecules onto what they produce. Think of the difference between your favorite shirt holding its shade after a dozen washes, or fading out in just two. That colorfastness doesn’t happen by accident—it depends on chemical reactions done right in the lab.
Pharmaceutical makers often look for acids that can kick off reactions and fine-tune product purity. With m-cresol sulfonic acid, synthesis specialists swap one chemical group out for another, drilling down to the mix they want. For certain drugs and specialty additives, this acid can tip the balance, making medicines safer and more effective. That kind of tuning matters—impurities and off-target reactions mean recalls, wasted investment, and real health risks.
Rust eats away everything from cars to factory parts. This acid finds a job in making anti-corrosive coatings and specialty surface treatments. The coatings industry leans on it to craft molecules that stop water and air from breaking down iron or steel. Old bridges and train tracks don’t stand up to the weather by luck. Underneath the paint, there’s real chemistry helping our infrastructure last.
M-cresol sulfonic acid does a lot of good but carries risks too. It’s caustic and corrosive, so careless handling leads to burns or worse for workers. Air and water pollution can become real headaches if plants cut corners. Regulation and tough enforcement tip the balance back toward safety. Switching to safer substitutes gets plenty of buzz, especially in Europe, but upgrading equipment or changing recipes doesn’t come cheap. The question always circles back to cost versus benefit—and who pays when something goes wrong.
Automation, better training, and advanced safety gear lower risks for people working with tough acids like this. Companies use real-time leak detection and stricter tracking of every batch shipped and used. Research teams keep chasing softer, greener alternatives, searching for a win-win: the same muscle in manufacturing without the pitfalls for workers or the environment. Real progress shows up in lower chemical spills, cleaner rivers, and safer jobs, but only if industry leaders keep investing and updating their methods.
M-Cresol Sulfonic Acid plays a part in making dyes, resins, and even pharmaceuticals. It brings plenty of power as a sulfonating agent but comes with tough storage demands. Most folks who handle chemicals know you can’t take shortcuts. One problem pop up and damage snowballs: health worries, property damage, and big costs to clean up.
No one wants to see rust spreading in their chemical warehouse. M-Cresol Sulfonic Acid acts fast on metals like iron, tinplate, or aluminum. Tanks or drums made with these materials break down, so storage tanks and piping need to use specially coated steel or lined plastics—think PTFE, HDPE, or glass lining for big setups. Industry experience backs this up; companies like BASF and Lanxess report using glass-lined steel for all transport and storage, keeping repairs and downtimes to a minimum over long stretches.
Heat picks fights with acids. Many people think only fire is a problem, but a warm room speeds up breakdown and vapor release. Strong acidic fumes end up eating away at seals and joints. Records from the National Institute for Occupational Safety and Health (NIOSH) show that poorly cooled or sun-exposed containers sent employees for medical checks far more often than those in consistently cool areas. It’s best to keep the acid below 25°C, with 15–20°C minimizing strike risks. Underground or shaded chemical storage helps fight off temperature swings and UV rays.
Water isn’t a friend. Once moisture sneaks in, M-Cresol Sulfonic Acid gets runnier and loses quality fast, reacting to form byproducts or causing gelling that clogs processing lines. Tightly sealed lids and air-locked drums aren’t just suggestions, they stop whole batches from spoiling. Some chemical engineers place silica gel packs in barrel storage—not as overkill, but because testing shows moisture creeps in even during short-term use if left unchecked.
Labeling isn't about red tape; it’s about keeping everyone in the loop. Drums need clear, chemical-resistant labels showing acid concentration and hazard icons. This way, nobody mistakes one barrel for another. Over several years in manufacturing, I’ve seen quick, clear labeling prevent close calls. Good ventilation in storage areas can clear out accidental fumes, and having chemical splash goggles and acid-resistant gloves nearby isn’t just policy—it’s practical. Proper first aid kits and neutralizing agents, like sodium bicarbonate, belong close to storage spots. It’s not just regulations, but peace of mind in a pinch.
Companies like Merck and Sigma-Aldrich keep their acid inventories locked up in dedicated chemical cabinets, with spill trays and fireproof walls. It’s not only about following a checklist; it’s about respecting a substance that can cause real harm. Real stories from seasoned staff highlight how skimping on acid storage ends up with blown seals, ruined batches, or worse, burns and lung injuries. Taking time to set up secure shelving, routine checks for container swelling or leaks, and spill drills with the whole team cuts risk down before accidents ever get the chance.
Industrial-scale plants and small workshops come up against the same basic lesson—the safest setup comes from combining good materials, steady temperatures, dry conditions, proper gear, and crystal-clear instructions. Making these habits part of the workplace culture keeps everyone and everything protected, week in and week out.
Standing in front of a chemical drum brings up memories from my early days on the plant floor. The smell of certain strong acids—one of which is m-cresol sulfonic acid—sticks in the mind. That sharp, biting odor tells you it’s not something to treat lightly. In manufacturing and labs, this compound often plays a role in producing resins, dyes, and other chemicals, so a lot of workers have seen its name on shipping labels. Regular contact isn’t rare in many parts of industry.
Working with m-cresol sulfonic acid without proper gear invites trouble. The acid is highly corrosive. Get any of it on bare skin or in the eyes, and the result can be fast, painful burns. Once, a co-worker splashed a tiny drop on her arm, and she didn’t notice right away. Minutes later, her skin began to sting and blister. These hazards aren’t just limited to burns. If inhaled as a mist or vapor, it irritates airways, sometimes leading to coughing, chest tightness, or even breathing problems. Swallowing it—even a small amount—brings on rapid, severe internal damage.
Sensitivity doesn’t end there. Some people develop all-over rashes or respiratory reactions after repeated exposure. I remember one tech who started getting headaches and runny eyes on days when the acid drums came in. Over time, lower-level exposures may add up, and chronic contact with phenolic compounds sometimes puts stress on the liver, kidneys, and nervous system.
M-cresol sulfonic acid doesn’t just harm people. Factories sometimes struggle with wastewater handling because releases of this acid endanger aquatic environments. Fish die-offs have happened in places where spills went untreated. Soil contaminated with these chemicals doesn’t support plant life easily. The chemical’s persistence means it hangs around unless managed carefully.
Complacent attitudes hurt safety. Trust the wrong gloves or skip the goggles, and accidents come quickly. Good practice means ensuring chemical-resistant gloves, aprons, and full face shields are standard whenever handling m-cresol sulfonic acid. Real leaders check the Material Safety Data Sheet before every transfer. It’s not about following rules but making sure everyone goes home in one piece.
Ventilation matters in any workspace handling harsh acids. Closed transfer systems and fume hoods keep the air safe. Training staff to spot risks, recognize early symptoms, and act quickly helps avoid small mishaps turning into emergencies. Clear emergency showers and eyewash stations should never get blocked. In places I’ve worked, tight housekeeping saves plenty of incidents over time.
Substitution brings direct results where possible. Safer acid alternatives make sense if the process allows for it. Adjusting workflows, using automation, or changing product formulas can remove the human factor from high-risk areas entirely. It takes investments, but they prevent costs from injuries and lost productivity in the long run. Good engineering, clear communication, and real accountability always beat wishful thinking. Chemicals like m-cresol sulfonic acid demand respect, not shortcuts.
M-Cresol sulfonic acid isn’t a household name, but plenty of folks in labs and factories come across it. The stuff eats away at skin on contact. Breathing in the vapor brings a stinging feeling in the nose, mouth, and chest. If it splashes in your eyes, the damage can be permanent and very painful. I remember a story from the plant floor: one technician slipped on a wet patch during a routine transfer, and a single drop burned right through two layers of gloves. After that, nobody skipped the thicker neoprene gloves again.
Basic nitrile gloves just don’t cut it for chemicals like this. Rubber or neoprene gloves are a much better choice. Every person handling the acid should also wear long sleeves, chemical-resistant aprons and face shields. Goggles are bare minimum. In my first years in the field, I saw too many accidents traced back to someone grabbing the closest gloves instead of the right pair. Shortcuts nearly always added up to skin burns and lost days at work.
Even small exposures to the vapor sting your nose and eyes, and cause a burning feeling down your throat. Open windows aren’t enough. Local exhaust—like a chemical fume hood—pulls the fumes away right at the source. I once watched a production run in a shop with poor ductwork, and half the crew complained about sore throats by the end of the shift. Smart managers keep air moving, so nobody has to risk their lungs on the job.
A few times, I’ve seen confusion turn a small spill into a big mess. Folks often grab a mop and a bucket of water, trying to act fast. In reality, water only makes things worse; it can splash the acid, spread fumes, or sometimes cause heat. Absorbent pads meant for acids work far better than paper towels or old rags. After every cleanup, the used pads and broken containers end up in sealed bins, labeled sharp and clear.
Writing a safety manual isn’t hard. Teaching people the reasons behind it takes more effort. Direct experience helps drive home what can go wrong. I’ve found that regular, hands-on drills help new staff remember the right steps, not just memorize pages of instructions. Quick, clear walk-throughs with senior folks on the team make a big difference for new hires and old hands alike.
Leaving containers unsealed leads to leaks and strong smells. Shelves should hold chemical-resistant trays to catch any drips. M-Cresol sulfonic acid also reacts strongly with bases and oxidizers, so smart storage means keeping it away from other reactive substances. Locking up acid cabinets during off-hours stops well-meaning but untrained staff from stumbling into trouble.
Everyone in the workplace ought to know the location of the nearest eyewash station and safety shower. In a pinch, wasting seconds looking for help isn’t an option. I’ve seen fire extinguisher drills save both lives and days of production after a chemical mishap. Keeping emergency numbers posted in plain sight means faster responses and better outcomes.
Handling M-Cresol sulfonic acid with care only works as well as the real-world habits of the crew. Good gear, smart storage, pride in preparation, and a willingness to coach each other—those are what keep workers safe day in and day out. Every workplace sets its own safety culture through countless small decisions, and for this chemical, those decisions matter every single day.
Nobody wants to discover that a pricey batch of chemicals has gone bad. M-Cresol Sulfonic Acid lands on purchase orders across labs and manufacturing lines. This sulfonic acid, known for its strong acidity and use in dyes, plastics, and resins, isn’t immune to age and environment. Every chemist learns one painful lesson: storage conditions make or break product quality.
Feeding off what’s written in Safety Data Sheets, manufacturers typically give untreated, unopened M-Cresol Sulfonic Acid about 24 months before worries about stability creep in. Yet, printed dates don’t always reflect ground reality. One person’s solvent cabinet at 18°C stays cool and steady. Another stashes containers in a steamy plant storeroom. Opened drums, leaky lids, or sitting near sunlight? All those shortcuts speed up change—color, smell, or crystal formation reveal degradation risk faster than any certificate.
People often shrug at shelf life, thinking strong acids resist everything. Truth is, this mindset creates big headaches. M-Cresol Sulfonic Acid absorbs water from air, then breaks down to byproducts and loses effectiveness. One year’s waste can cloud years of results in quality control labs. I saw a small production site ignore their stock for too long. Resin batches wouldn’t cure right, costing a few thousand dollars in time and out-of-spec batches. Just one poorly maintained drum can bring a halt to an entire line.
The health risk matters, too. Older acid stings sharper; it might build up impurities or release toxic fumes. Handling risks grow with time. Every workplace accident report I’ve read that ties back to chemicals past their prime could have been avoided with routine checks or better labeling.
The easiest win is proper storage. Seal drums tightly right after use. Keep containers dry, out of direct light, and under about 25°C. Letting acids heat up or freeze cracks seals, compromising the contents. Rotate stock—older containers move forward, new goes to the back. Checking labels and making a log keeps confusion at bay, especially in shared labs or warehouses.
Paying attention to signs of deterioration matters more than inked expiration dates. Any change in color or the appearance of crystals or sediment signals time to test or dispose. Training staff to check for these signs, to spot off smells, and to never combine old and new acid saves far more than good intentions ever will.
Asking suppliers for stability data and specific handling tips clears up common misunderstandings. Some even provide testers or small aliquots for older stock. Third-party testing can confirm acid strength and purity—long before any major project kicks off.
Every time I check M-Cresol Sulfonic Acid storage, I remember stories of wasted inventory and ruined batches. Respecting its shelf life, labeling, and keeping it away from humidity always paid off. No shortcuts or guesswork, just steady records and regular monitoring. Shelf life isn’t just a number; it’s the line between smooth production and last-minute audits or recalls. Following these steps puts real-world dependability ahead of dusty bins and second guesses, keeping people safe and business running strong.
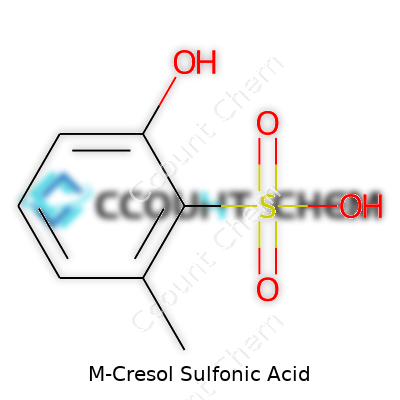
| Names | |
| Preferred IUPAC name | 3-Methylbenzenesulfonic acid |
| Other names |
m-Cresol Sulfonic Acid 3-Cresol Sulfonic Acid 3-Hydroxy-toluene-4-sulfonic acid meta-Cresol Sulfonic Acid m-Cresolsulfonic acid 3-Methylphenol-4-sulfonic acid |
| Pronunciation | /ɛm-ˈkriːsɒl sʌlˈfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | # 118-64-5 |
| Beilstein Reference | 3678924 |
| ChEBI | CHEBI:28664 |
| ChEMBL | CHEMBL115470 |
| ChemSpider | 2030056 |
| DrugBank | DB11274 |
| ECHA InfoCard | 100.007.943 |
| EC Number | 215-539-3 |
| Gmelin Reference | 8785 |
| KEGG | C11372 |
| MeSH | D003437 |
| PubChem CID | 8717 |
| RTECS number | SN6475000 |
| UNII | 4S3300R575 |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID6022172 |
| Properties | |
| Chemical formula | C7H8O4S |
| Molar mass | 172.18 g/mol |
| Appearance | Brownish yellow transparent liquid |
| Odor | Phenolic odor |
| Density | 1.27 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -0.41 |
| Vapor pressure | <0.1 mmHg (20°C) |
| Acidity (pKa) | 1.31 |
| Basicity (pKb) | 10.09 |
| Magnetic susceptibility (χ) | -5.1×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.572 |
| Viscosity | 100-200 cP |
| Dipole moment | 3.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 192.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -487.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3565 kJ/mol |
| Pharmacology | |
| ATC code | D08AX04 |
| Hazards | |
| Main hazards | Corrosive, causes severe skin burns and eye damage, harmful if inhaled, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 |
| NFPA 704 (fire diamond) | 3-0-2-Acidos |
| Flash point | 89 °C |
| Lethal dose or concentration | LD50 Oral Rat: 641 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1600 mg/kg (oral, rat) |
| PEL (Permissible) | PEL: 5 mg/m³ |
| REL (Recommended) | 3 mg/m³ |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
Cresol p-Cresol sulfonic acid Benzenesulfonic acid o-Cresol sulfonic acid Toluene sulfonic acid |