Chemistry tends to blend the past and present, especially when a compound like m-cresol-6-sulfonic acid ammonium salt enters the story. Decades ago, chemists stumbled onto the cresols as they searched for ways to improve dyes, disinfectants, and various organic syntheses. The sulfonation of cresols transformed their basic utility, opening up a path to industrial intermediates that would find work in everything from resin manufacturing to fine chemicals. Ammonium salts of these acids emerged as a solution for better solubility and storage stability. Researchers dug deep into the reaction dynamics and process controls, gradually honing methods to produce cleaner, more consistent batches. The story is less about grand discovery and more about persistent tinkering and practical goals. Most of the early work took place in crowded laboratories powered by glassware, gas burners, and hard-earned experience, not by luck or happenstance. Each improvement grew out of daily problems: product loss, unpredictable contaminant levels, and the feverish rush to scale up when a process finally worked.
M-cresol-6-sulfonic acid ammonium salt is valued for its role as a versatile intermediate. Chemists gravitate toward it when exploring applications in dyestuffs, pharmaceuticals, and specialty chemicals. The salt handles conveniently—even in humid labs. Its relatively high melting point and crystalline structure help with storage and transport. Formulators see this compound as a practical choice; it's not the flashiest, but it gets the job done without the baggage that some related chemicals drag along. Purity varies depending on the end use, but tighter tolerances show up in pharma work, while broader grades suit industrial synthesis. Packaging usually features clear hazard labeling, meant to keep workers on their toes and regulators satisfied.
The salt forms white to off-white crystals, sometimes showing a faint yellow tint if not protected from air and light. It dissolves easily in water—one of its more helpful traits. The melting point often lines up in the range of 240–260°C, depending on trace impurities carried forward from its synthesis. It emits a faint phenolic odor. In the lab, I always noticed how the compound showed surprising chemical resilience. It resists gentle oxidation, doesn’t decompose immediately when exposed to atmospheric humidity, and remains compatible with a variety of solvents. Under stress from heat or strong acids, it can release ammonia or phenolic fumes, which the nose detects faster than any meter.
Suppliers list minimum assay levels, commonly above 98%. Heavy metals, chloride, sulfate, and water content face close scrutiny as these trace constituents influence downstream processes. Labels cover chemical identity, safety instructions, and UN GHS hazard statements like ‘harmful if swallowed’ or ‘Causes serious eye irritation.’ Packaging includes tightly sealed plastic or glass containers to cut down moisture uptake. Once, I saw a shipment rejected at a pharmaceutical site because the salt had clumped due to poorly sealed packaging—reminding everyone that technical specifications mean little if storage practices falter.
Production usually starts with m-cresol, sourced from coal tar or petroleum streams. Sulfonation generally comes next, using fuming sulfuric acid or chlorosulfonic acid. The reaction takes careful monitoring—overdoing it leads to poly-sulfonation and byproducts that are tough to remove. Neutralization with aqueous ammonia completes the process, forming the ammonium salt and scrubbing residual acid. Crystallization requires cold temperatures and slow addition of ammonia. Early on, failed crystallizations created sticky messes thanks to careless cooling or incomplete reaction. Lessons learned: patience, precise acid-to-cresol ratios, and slow addition yield a cleaner product. Filtration and repeated washing produce the pure white salt favored by most technical standards.
This salt’s sulfonic acid group serves as a handle for more complex chemical building. It can undergo substitution reactions, further sulfonation, or coupling with amines or aromatic rings. Researchers often use it to introduce sulfonic functionality into target molecules. The ammonium moiety makes it less corrosive and easier to handle compared to the free acid, especially in water-based reactions. Chemical reductions, oxidations, and condensations can proceed under milder conditions—in some cases, the ammonium salt serves as a phase transfer catalyst. One memorable project involved modifying the salt to build a new family of non-ionic surfactants used in high performance lubricants. Problems often appear in purification, so I always recommend small-scale trials to iron out pathways before scaling up.
Chemists call this salt by many names: 6-sulfo-m-cresol ammonium salt, ammonium 6-hydroxy-3-methylbenzene sulfonate, or just plain 'ammonium m-cresol sulfonate' on shipping manifests. International suppliers tack on catalog numbers and company-specific designators, but the chemical backbone remains unchanged. Trade names sometimes pop up in industrial circles, but regulatory authorities always push for IUPAC nomenclature to cut confusion.
Lab safety officers stress the importance of avoiding contact with skin or eyes. The compound does not give off clouds of toxic vapor under normal use, but dust or excessive heat will release ammonia, which stings the nose and throat on contact. Standard PPE—nitrile gloves, lab coat, tight-fitting goggles—always stays within arm’s reach during my sessions with this salt. Ventilation makes day-to-day handling less risky, and dedicated waste containers mean accidental mixing remains rare. MSDS fact sheets require regular review, and it always pays to assign clear responsibilities during bulk transfers to prevent mishaps from inattention or fatigue.
Dye and pigment manufacturers use this compound as a coupling agent to introduce sulfonic acid into aromatic rings, helping dyes dissolve better in water. Pharmaceutical chemists sometimes rely on its reactivity to build more complex intermediates. Water treatment operators find it useful as a scale inhibitor thanks to its sulfonic functionality. Polymers and specialty resin producers select it for controlled release and ion exchange properties. My experience with lab-scale pilot lines revealed how small tweaks in process conditions drive product yield and purity, which then show up as cost savings across long production runs.
Research teams continue to explore new uses, especially in greener chemistry. The salt’s solubility and reactivity make it a candidate for replacing harsher reagents in some processes. Collaborative projects aim to pair this compound with emerging technologies, such as membrane separations and micron-scale catalysis. Universities run toxicology and environmental fate studies to ensure that scale-up will not trigger new headaches for safety or compliance. I joined one group effort on biocatalytic modification, where we probed for routes to degrade or transform sulfonated aromatics using enzymes—progress moves slowly, but the goal is lower energy inputs and fewer hazardous byproducts.
Animal studies and cell assays indicate moderate acute toxicity, mostly tied to the cresol core, which affects nervous system function if mishandled or ingested. Chronic exposure links to liver and kidney stress, although the ammonium counter-ion lowers the risks compared to free acids. Environmental fate investigations show that microbes break down the compound slowly, especially in waterlogged soils. This slow degradation highlights the need for careful disposal and the risk of accumulation, particularly if large quantities get into waterways. I’ve seen plant operators tighten spill response rules as new data points roll in from risk assessments. Proper training reduces accidents, and ongoing studies hint at safer workarounds as alternatives mature.
Production facilities worldwide seek improvements with greener raw materials and less hazardous reaction conditions. Shifts toward stricter regulation and increased demand for safer intermediates drive research into cleaner synthesis. Some chemists are working on enzyme-based sulfonation methods to reduce both waste and emissions. Automation enters the scene—inline sensors trim out-of-spec batches before they reach the downstream steps, letting workers focus on higher-order troubleshooting. Material scientists push modifications that broaden application in battery technology and sustainable plastics. Those of us close to production and lab innovation often see tomorrow’s opportunities in today’s headaches—the more we study this ammonium salt, the more we spot practical roles in the transition toward safer, more sustainable chemistry.
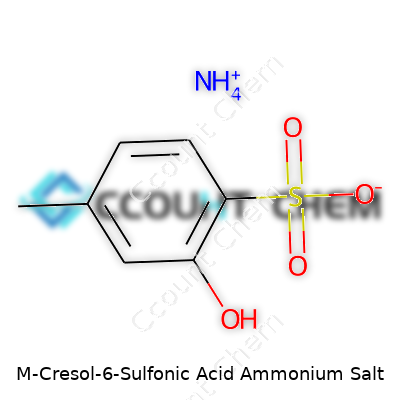
M-Cresol-6-sulfonic acid ammonium salt isn’t a name you bump into walking through a supermarket. The chemical formula often gets written as C7H9NO4S. That formula sketches out a benzene ring with a methyl group in the meta position, a sulfonic acid, and an ammonium salt holding everything together. For people like me who have worked around chemical stockrooms and research, this sort of structure isn’t just a string of letters and numbers. Each part plays a role in how the compound behaves.
I’ve watched labs grind to a standstill over the tiniest details in a formula. A single misplaced atom or ion can turn a chemical from a useful tool to an irritant, or worse, to something toxic. That’s one of the reasons why the chemical structure C7H9NO4S matters. The ‘m-cresol’ means the methyl group attaches at the third carbon, not just anywhere. The ‘6-sulfonic acid’ tag shows that the sulfonic group hooks onto the benzene at the sixth carbon, giving the compound a particular reactivity and solubility. The final touch, ammonium, anchors the sulfonic acid in a salt form, making it less acidic and more amenable for use in various syntheses.
Folks working in dyes, pharmaceuticals, and industrial chemistry see this salt crop up more often than you’d think. The ammonium salt pops up as a building block in dye manufacturing, and sometimes in the background of pharmaceutical syntheses, helping to create pigments or other needed chemicals. Each tweak to the formula can mean a batch works as planned—or doesn’t. Years ago, I remember a shipment coming in labeled only by the generic name, missing the salt. That small omission fouled up an entire synth run, costing the team days. Getting the formula right at this stage keeps projects from going off the rails.
Safety sheets stress handling compounds like M-cresol-6-sulfonic acid ammonium salt with gloves and goggles. With exposure, the sulfonic part is mild compared to some chemicals, but the cresol ring doesn’t play nice with skin or lungs in large doses. Data from the CDC shows cresol compounds, if managed poorly, can lead to respiratory issues and skin burns. Keeping the chemical in salt form with ammonium dulls a bit of the risk, as it won’t outgas or degrade as quickly as a pure acid, but it shouldn’t lull anybody into carelessness.
Experience in the lab teaches that proper labeling, training, and storage saves headaches later. Digital inventory systems make double-checking the formula routine, so mistakes fade into the past. For busy operations, pre-weighed and sealed containers cut down on handling mistakes. Pairing technical know-how with habits like routine tool checks, fresh gloves, and solid fume hoods increases confidence in handling. Researchers gain by sharing error stories and safety updates so the next set of hands learns from today’s problems.
Once the composition is known, the focus shifts to how the material helps or hinders the work. Consistent supply, clear documentation, and a tight feedback loop between lab workers and suppliers reduce headaches and costs. The world of chemical manufacturing and research doesn’t thrive on shortcuts. Precision matters in every step—from the moment a delivery arrives to the last data point recorded on the bench. The right formula opens possibilities, but experience and vigilance set the real boundary between success and setback.
M-Cresol-6-sulfonic acid ammonium salt doesn’t get much attention outside of lab circles, but if you look into certain chemical processes, this compound has proven itself useful in several ways. I remember mixing similar aromatic sulfonic acids in the lab during university, trying not to spill anything since my professor cared more about safety than getting the experiment done quickly. Real-world work isn’t much different—people rely on these salts for their reactivity, not their looks.
Few things in our homes feel as ordinary as the colors on clothes or paper, but those colors come from materials made through careful chemistry. M-Cresol-6-sulfonic acid ammonium salt acts as an intermediate here, helping bind colorants more stably onto fibers or paper. It brings sulfonic groups that improve solubility and the sticking power of dyes. Manufacturers use it for azo dyes and certain pigment preparations, especially when they want lasting color that doesn’t fade fast. The same chemistry that keeps your favorite sweater vibrant pulls a lot of weight behind factory doors.
Every time I look at a common medication, I think of the layers of reactions that built its complex form. Chemical building blocks like m-cresol-6-sulfonic acid ammonium salt help piece together complicated pharmaceuticals. Drug makers reach for compounds like this when they need to pull electrons or rearrange molecular backbones, making reactions run smoother or cutting down on byproducts. According to the American Chemical Society, improved efficiency and fewer side products matter in both costs and environmental impact. Simple, reliable chemistry behind the scenes contributes to the shelves of medicine at your local drug store.
Materials science depends on the right balance between flexibility, toughness, and chemical resistance. By incorporating compounds with sulfonic acid groups, like m-cresol-6-sulfonic acid ammonium salt, companies can make resins that handle heat or aggressive chemicals better. These tweaks help extend the life of coatings, electrical laminates, and adhesives. I’ve seen engineers choose sulfonated resins for circuit boards that get baked in manufacturing lines—details like that have more to do with chemical tweaks than fancy electronics.
Fresh water hasn’t gotten easier to come by. Some treatment plants add aromatic sulfonic acids to help remove metal ions or break up organic sludge during the treatment process. The way these salts grab hold of contaminants offers a practical advantage for operators under pressure to keep water safe and clear. Published studies from Water Research Journal back up these techniques, confirming practical gains over traditional methods, especially for dealing with industrial runoff.
Any time a strong acid or chemical salt makes its way into production, questions about health and the environment come up for good reason. Handling m-cresol-6-sulfonic acid ammonium salt involves plenty of protective gear to avoid skin or lung irritation. Up-to-date safety training forms the backbone of every chemical plant visit I’ve done, and regular audits help catch issues before they affect workers or the outside world. Switching to greener alternatives calls for collaboration between researchers, manufacturers, and regulators, aiming for the same performance with less hazard.
Anyone who’s spent time around labs or warehouses knows that what gets stored on shelves isn’t always harmless. One wrong move can turn a routine task into an emergency. Take it from years spent in places where a spill could shut down a whole wing. A lot of people treat storage like stacking cans in a cupboard, but every chemical has its quirks—some react to air, others get dangerous if they heat up. Sticking a drum in the wrong corner or using the wrong container can set off a chain of problems nobody wants.
For most chemicals, conditions like temperature, light, and humidity are not just optional afterthoughts. Storing something like hydrogen peroxide or nitric acid close to a heat vent, or in the path of sunlight, can nudge them into breaking down or boiling. Heat speeds up reactions. That’s how courts end up with cases about ruined shipments or, worse, warehouse explosions. Keeping temperature consistent, away from direct sunlight, plays a big role in keeping chemicals stable for as long as possible.
The best-organized labs and storage rooms have every bottle, drum, and container clearly labeled. Names, concentrations, expiration dates, and hazard symbols show anyone—whether it’s a veteran scientist or a new crew member—what’s inside. This isn’t about ticking boxes for an audit. Labels stop accidents, make emergency response smoother, and keep people from reaching for the wrong thing at the wrong time.
I’ve watched new staff toss bleach and ammonia on the same shelf more times than I can count. These chemicals seem harmless enough on their own, but if they spill and mix, you get toxic gas. Acids and bases stay apart for a reason. Flammables belong in special cabinets, preferably metal and vented, never next to oxidizers. Solvents need their own space. Mixing things up isn’t just a mistake—it’s inviting hazards through the front door. Segregation matters because every chemical can play by different rules, and not every reaction announces itself before it’s too late.
Shoddy containers—cracked, badly sealed, or made from the wrong material—lead to leaks and contamination. Some chemicals chew through plastic, while others corrode metal. For acids, glass often works best. Strong bases or solvents usually call for HDPE or metal, though it depends on the specific properties. Regularly checking for leaks or bulges in containers can help spot trouble early. I’ve seen labels fall off sweaty bottles, and that confusion can get someone hurt. Good practice suggests sticking to original packaging or containers rated for hazardous goods, and always replacing damaged parts right away.
Goggles, gloves, and sturdy lab coats serve as barriers, but overreliance can give a false sense of confidence. Letting your guard down on storage or ignoring handling rules just because you’re suited up misses the point. Proper PPE reduces risks, not eliminates them outright. Training counts for a lot, too. People should know what to expect with each chemical, from what the Safety Data Sheet (SDS) outlines to how to respond if something goes wrong. Regular drills and refreshers can keep good habits sharp, especially for teams dealing with multiple substances every day.
No one should feel too proud or busy to check the SDS. These sheets layout what can go wrong and what to do if it does. Online resources, direct calls to manufacturers, or consultations with occupational safety experts can add an extra layer of confidence. One thing is clear: Doing what’s right for chemical safety takes more than locking doors and hoping for the best. It runs on clear information, careful habits, and a little skepticism about shortcuts.
M-Cresol-6-Sulfonic Acid Ammonium Salt shows up in more chemical labs than the average person might guess. This isn’t some household cleaner or an ingredient you’d find in a food additive. Folks handling this compound should know its risks straight from the safety data sheets and firsthand stories shared across industry networks. Speaking from experience, working with chemicals brings a heightened sense of awareness. The way you treat a beaker, how you store materials on a shelf, the conversation you have about a new shipment—every detail matters.
If you spill this compound, things can get messy fast. Its corrosive qualities can cause serious burns to skin and eyes. Direct contact doesn’t just sting—a careless moment can land someone in the emergency room with serious tissue damage. Splashes to the eye, in particular, can mean permanent loss of vision. Gloves and goggles aren’t just for show in a lab; they’re basic survival tools. Even a small accident can turn a normal day into a rush to the eyewash station.
Breathing in dust or fumes raises another set of problems. The ammonia present can irritate the lungs and throat. No one in the lab forgets their first breath of ammonium vapor—the sharp burn, the sudden coughing. Irritated mucous membranes lead to bigger health challenges after repeated exposure, including headaches, chronic respiratory irritation, and more. I’ve watched experienced chemists get tripped up by shortcuts, choosing speed over safety, and the resulting mistakes can linger a long time.
Spills are only one angle of risk. Storing this salt with incompatible materials can spark unexpected reactions, including toxic gas formation. Mixing it, even by accident, with strong oxidizers or acids causes dangerous fumes to fill the air. I learned from an early mentor to double-check every container in the storage room because one mislabeled jar could cause catastrophe. The value of clear labeling, locked cabinets, and a disciplined inventory system grows clear with every lab inspection or close call you hear about.
Waste disposal creates additional headaches. Pouring leftover or expired salt down the drain or letting it get into the general waste stream isn’t just bad for the environment—it tends to violate local laws, too. Water systems and landfill workers can get exposed to corrosive byproducts or noxious gases long after the material leaves a lab. Companies who cut corners on disposal end up paying for it in fines and damaged reputations.
An old lab tech once told me, "If you think you can skip the safety gear, you haven't seen a real accident yet." That line never left me. Proper training is non-negotiable. Every new crew member needs to learn spill response, accident first aid, and the right storage rules from the start. Standard operating procedures built from years of lessons from incidents and near-misses keep everyone alert.
Ventilation systems go a long way toward protecting lungs, and personal protective equipment sets the line between routine and disaster. Getting buy-in from the full team comes down to sharing real-life stories and making the risks plain to see. Regular drills, clear communication, and investment in up-to-date storage solutions keep incidents from wrecking lives. Anyone who puts safety first sees fewer injuries, tighter regulatory compliance, and stronger trust among every worker who clocks in.
Purity makes a real difference. In my years of dealing with industrial chemicals and basic lab supplies, I’ve learned that people want details you can trust. Purity isn’t just a number—it affects performance, storage, safety, and sometimes the law. Product quality statements like “99.9% pure” mean more than just a badge on a label. For food additives and pharmaceutical ingredients, even tiny impurities can change the outcome, sometimes in life-or-death ways. A mechanic or an engineer wants a cleaner, more precise result when using high-purity solvents or reagents, and so do folks running small businesses who can’t afford to gamble on unknowns.
Government regulations add another layer. The European Union, FDA, and other bodies all set legally binding thresholds. Trace metals, unknown contaminants, or strange odors quickly invite unwanted inspections or product recalls. Sometimes, a product needs to be certified “food grade,” “USP grade,” or “analytical grade,” and the difference is more than just paperwork. For something like sodium chloride, food processors expect 99.5% or greater (by weight), with even smaller tolerances in laboratory work. On the farm, fertilizer purity comes up less, but seeds and soils worth their salt still need some guarantee.
Too often, companies assume buyers want large containers. In my early days, I worked with start-ups that wasted money on drums they couldn’t store, losing product to spoilage. Others fought leaking or awkward canisters. Experiences like these underline the fact: package size makes a difference. Home bakers and classroom teachers want small bottles or sachets. Concrete plants, bakeries, and cleaning services have different storage needs altogether. Hospitals like to see sealed ampoules, smaller vials, or clearly labeled pouches so there’s minimal risk of mistakes.
It helps to picture the range. Bulk buyers—think big food manufacturers or chemical processors—lean towards 25 kg bags, large fiber drums, or intermediate bulk containers (IBCs) that look like giant plastic cubes on pallets. Small businesses and labs need 1 kg jars or 500 g bottles that are easy to shelve and keep dry. Hobbyists, teachers, and maintenance crews look for single-use packets, maybe 50 to 250 grams, especially if there’s only occasional need. Sometimes, we see companies selling sample sets, 10 g or less, for research or product qualification.
Low-quality product hurts people and reputations. I learned early that poor labeling or confusing size charts often led to mistakes—not a good outcome for anyone. Clear statements on a product page or label, listing both the purity percentage and all available packaging sizes, save headaches later. Some websites go a step farther with certificates of analysis or safety data sheets. These documents give real facts, not just marketing fluff, and help everyone from purchasing managers to students understand exactly what they’re buying.
There’s another angle to think about: sustainability and waste. Smaller packaging fits small needs, so less ends up in the trash or wash-down drains. Bigger bulk containers cut down on plastic waste and transport costs, especially for large buyers. Companies should offer several size options, with clear guidance on how to store or dispose of leftovers.
Finding the right mix of purity and package size isn’t hard, but it does require clear, up-front information. Suppliers need to spell out what’s on offer, with numbers that mean something outside the lab. Buyers do best when they match these specs to real-world needs—saving money, avoiding waste, and staying out of trouble with quality inspectors. Brands that show off their purity data and packaging line-up, instead of hiding them, earn more repeat business and happier customers.
| Names | |
| Preferred IUPAC name | ammonium 3-methylphenol-6-sulfonate |
| Other names |
Ammonium 3-methyl-6-sulfophenolate Ammonium m-cresol-6-sulfonate |
| Pronunciation | /ɛmˈkriː.sɒl sɪks sʌlˈfɒnɪk ˈæsɪd əˈmɒniəm sɔːlt/ |
| Identifiers | |
| CAS Number | [13124-38-0] |
| 3D model (JSmol) | Here is the **JSmol 3D model string** for **M-Cresol-6-Sulfonic Acid Ammonium Salt**: ``` CC1=CC=S(=O)(O)C=C1N.[NH4+] ``` *This is the SMILES string used for JSmol 3D visualization.* |
| Beilstein Reference | 607968 |
| ChEBI | CHEBI:91249 |
| ChEMBL | CHEMBL1882086 |
| ChemSpider | 56557 |
| DrugBank | DB13794 |
| ECHA InfoCard | 13d103ecc7b-48a6-4d4b-8d24-4a90c353e5e6 |
| EC Number | 247-639-6 |
| Gmelin Reference | 80777 |
| KEGG | C14346 |
| MeSH | D017967 |
| PubChem CID | 178697 |
| RTECS number | SN6470000 |
| UNII | 2O16AX4ZFC |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID50779988 |
| Properties | |
| Chemical formula | C7H9NO4S |
| Molar mass | 207.24 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.28 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -0.8 |
| Acidity (pKa) | pKa = 1.05 |
| Basicity (pKb) | 8.2 |
| Magnetic susceptibility (χ) | -64.2 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.528 |
| Dipole moment | 6.03 D |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH⦵298) | -878.4 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1,2,0 |
| Lethal dose or concentration | LD50 Oral Rat 700 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 2322 mg/kg (rat, oral) |
| NIOSH | Not listed |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for M-Cresol-6-Sulfonic Acid Ammonium Salt is not specifically established by OSHA or ACGIH. |
| REL (Recommended) | 12-18°C |
| IDLH (Immediate danger) | IDLH not listed |
| Related compounds | |
| Related compounds |
M-Cresol M-Cresol-6-Sulfonic Acid P-Cresol-4-Sulfonic Acid Ammonium Salt O-Cresol Toluene-4-sulfonic acid ammonium salt Phenol Benzenesulfonic acid ammonium salt |