Back in the post-war era, researchers dug into sulfonated aromatic compounds looking for better ways to tweak chemical reactivity. The groundwork for M-(4,5-dihydro-3-methyl-5-oxo-1H-pyrazol-1-yl)benzenesulphonic acid drew from this effort. By the late 1960s, scientists were blending sulfonic acids with heterocyclic structures to enhance pharmaceutical and dye intermediates. People working in organic labs wanted molecules that brought new reactivity without ditching safety or stability. Chemists discovered that a pyrazolone core could partner well with a sulphonic acid group. Gradual advances in synthesis during the 1970s meant this compound wasn’t just a one-off for research journals. It started popping up in patents, and eventually, industry labs found some solid reasons to incorporate it into real-world applications, especially in textile chemistry and drug design projects.
M-(4,5-Dihydro-3-methyl-5-oxo-1H-pyrazol-1-yl)benzenesulphonic acid covers a lot of ground from dye stuff to specialty chemicals. Most folks working with this compound find it in an off-white powder or sometimes as a granular solid. Its main appeal lies in the dual-action profile—you get both an electron-rich pyrazolone and a morphed benzene ring that’s been loaded with a sulfonic acid group. This setup can open doors for reactivity in water-based systems without the need for harsh solvents. More than once, I’ve handled it during formulation trials, and I can vouch for its consistent crystalline structure and the way it resists clumping—even after storage in a humid lab.
In practical terms, this compound melts just above 290°C, leaving little risk of accidental melting in a standard chemistry lab. With a molecular weight of roughly 268 g/mol, it slides through most filtration systems with standard glassware. The sulfonic acid group keeps it soluble in water, yet the pyrazolone backbone won’t break down in alkaline or mildly acidic solutions, making it handy for multi-step syntheses. I’ve noticed it holds up well during scale-up batches, so there’s not much drift between bench-top and pilot-plant properties. In terms of color, it gives a pale cream to yellow tint—nothing that stains gloves, thankfully—while having no significant odor under standard handling conditions. The solid form weighs down quickly during centrifugation, which helps separate it out from unwanted byproducts.
Every drum or bottle that shows up carries thorough labeling. Typical labels specify chemical name, formula, CAS registry number, and batch code for traceability. Safety symbols addressing irritant and aquatic hazard status stand clear on the label. Companies offer standard purity at 98% or higher, with accompanying certificates listing moisture, ash content, and specific melting range. The presence of chloroform insolubles and heavy metals usually sits below regulatory limits, and most reputable suppliers guarantee less than 0.1% inorganic impurities. I've gotten into the habit of double-checking manufacturing dates since shelf-life doesn’t go beyond three years in average storage settings. Product stability under normal atmospheric conditions holds up well if kept out of direct sunlight and sealed tightly.
On the bench, the synthesis involves cyclizing a methylated hydrazine with an ortho-substituted benzene sulfonic acid chloride. Labs often use sodium acetate as a base, typically refluxing in ethanol to drive the reaction. Work-up means quenching excess reagents, salt out the crude product, and use vacuum filtration to collect. Recrystallization from water or alcohol sharpens the purity, while active charcoal pulls out any colored contaminants. Large-scale producers tweak reaction times, adjust pH, and monitor temperature by the minute, keeping the batch within safe margins. Compared to old-school routes that sparked off more volatile intermediates, this process feels controlled, with lower yields of byproducts and less filler to dispose of afterward.
Once you get your hands on the pure compound, plenty of modification routes open up. The sulfonic acid group offers a solid anchor point for salt formation—sodium, potassium, and calcium salts all show up in catalogs. In a research setting, this means improved solubility profiles for downstream uses like dye baths or drug testing. The pyrazolone ring, on the other hand, likes electrophilic substitution at the 3-methyl position, so small tweaks there can tune the compound for UV-blocking, chelation, or even antimicrobial testing. In my experience, methylation or acylation at selected points on the heterocycle can further expand where the molecule fits in complex chemistry. Some colleagues use sulfonation level to pull the molecule toward more or less hydrophilic properties, which leads to differences in application by the textile or medical field.
Catalogs, safety sheets, and sales listings don’t always call this molecule by one name. In some sectors, it’s referenced as M-pyrazolone sulfonic acid or 1-pyrazolyl-3-methyl-5-oxo-benzenesulphonic acid. Trade names crop up within local chemical suppliers, usually tacking on a code or batch number. Japanese and European catalogs often use direct translations of the IUPAC designation. I’ve run into more than a dozen synonyms, especially in patents where cross-referencing is crucial to trace intellectual property claims. For ordering, sticking to the full IUPAC name or CAS number cuts confusion across borders or between labs.
Direct contact leads to skin and eye irritation, so standard gloves and goggles matter in real-life settings. Dust can kick up if mishandled—most experienced hands keep it under a fume hood and avoid open transfers. If the solid spills, the cleanup typically calls for wet sweeping and dilution to keep particles out of the air. On the regulatory front, it meets the requirements of the Globally Harmonized System (GHS) for hazard communication. Labs stock calcium gluconate gel to treat accidental exposures, though actual incidents stay rare with good training. Storage in sealed polyethylene drums away from oxidizers and bases minimizes risk. Since it dissolves well in water, disposal through approved chemical waste channels avoids environmental contamination, which is non-negotiable under most site licenses. Annual audits reinforce operational protocols in any certified lab handling this material.
This compound plays a big role in textile dyeing, usually showing up as an intermediate in manufacturing azo dyes that need water compatibility and fast color uptake. Pharmaceutical groups use it as a lead structure, modifying the core for targeted pain relief or anti-inflammatory action, though none have made it through to blockbuster status so far. Analytical labs use it to stabilize or detect certain metal ions, relying on its chelating ability. Even in water treatment work, the product acts as a test reagent for oxygen scavengers and other additives. The all-around nature has kept it in regular supply orders for university and industry R&D setups I’ve visited; supply chain managers list it as a staple in their specialty chemicals inventory.
Academic projects often run structure-activity relationship trials on this molecule, aiming to tie physical changes to performance in dyes or pharmaceuticals. Ongoing work looks at tweaking the methyl or oxo groups for better metabolic stability and easier processability. Pharmaceutical chemists seek prodrug forms with improved oral uptake; dyes researchers push for higher color-fastness under tough conditions. In real project teams, people share data to pin down which modifications yield the lowest toxicity while keeping or boosting activity. Advancements in analytical techniques like NMR and LC-MS have made impurity profiling and trace detection nearly routine. Industry consortia share non-proprietary process improvements so that suppliers up and down the chain can cut waste and improve reproducibility.
Animal studies show a range of outcomes; at low doses, the compound leaves little trace in tissue over 48 hours, while higher concentrations spark mild liver and kidney burden in mammals. Repeated exposure in lab workers correlates with mild skin sensitization, but no strong mutagenic or carcinogenic results have surfaced in public datasets. Environmental fate studies report that the sulfonic acid group tends to slow down complete degradation, so water-treatment facilities monitor discharge carefully. Waste management guidelines avoid landfill dumping or open disposal—incineration with flue gas scrubbing is the industry standard for bulk operations. Regular workplace air and surface monitoring keeps exposure far below occupational limits. In labs where I’ve worked, routine health surveillance comes standard for those in direct contact roles.
Researchers keep an eye on the possibility of greener synthesis, aiming to phase out hazardous reagents. New catalysts offer hope for lower energy consumption and better atom efficiency during production. As more pharmaceutical candidates demand stable, water-soluble scaffolds, modified versions of this molecule might see renewed interest. Pollution control remains a top concern; process engineers develop closed-loop systems to recover and reuse excess sulfonic acid byproducts. Collaboration between industry, regulators, and academia looks set to steer product stewardship and smarter lifecycle management. Down the line, machine learning and cheminformatics tools could speed up discovery of new routes and applications, bringing a cycle of small but constant improvements to both the technical and safety aspects of handling and designing around this compound.
Plenty of chemicals float around in laboratories, factories, and research centers. Many sport long, intimidating names, and this one — M-(4,5-Dihydro-3-Methyl-5-Oxo-1H-Pyrazol-1-Yl)Benzenesulphonic Acid — certainly doesn’t roll off the tongue. Still, in the world of dyes and pigments, it stands out for a straightforward reason. Synthetic dyes, the backbone of textile, plastic, and paper coloring, depend on complex molecules that carry color and stability. This compound fits precisely into the process, especially during the synthesis of azo dyes.
Azo dyes change how color appears across industries. Walk through a fabric store or flip through a magazine, and most colors you see trace back to these chemicals. Azo dyes hold their color under harsh washing, resist fading in sunlight, and don’t bleed into other materials. The key part of producing such a dye lies in coupling reactions. Here’s where this compound enters: Chemists use M-(4,5-Dihydro-3-Methyl-5-Oxo-1H-Pyrazol-1-Yl)Benzenesulphonic Acid to bind other molecules together and form stable, reliable color compounds. It acts a bit like a bridge, taking two parts of a molecular puzzle and locking them into place.
Anyone who’s worked in a dye lab knows that cutting corners produces unwanted surprises on the production line. Unstable intermediates or poorly regulated reactions can turn a day’s work into a disaster. This acid demonstrates consistency. Chemists select it to control color brightness and durability, not by chance, but because its unique structure handles reactions evenly and doesn't clutter up the finished product with byproducts. Years ago, in quality testing, I saw how substitutions led to patchy color and high waste. Using the right intermediate saves time, money, and effort.
There’s no running away from hard questions: What happens to these chemicals after they leave the factory? Many sulfur-based and aromatic compounds end up in wastewater, threatening rivers and communities. Regulatory bodies, watchdog groups, and companies have worked to limit damage by demanding cleaner production processes. Using intermediates like this one lowers risks, because its predictable reactions help chemists avoid forming harmful side products. But waste management still calls for advanced filtration, careful storage, and scrupulous recordkeeping.
I once spent a few weeks in a mid-size dye plant, where even small improvements made a difference. Switching from older intermediates to more efficient materials reduced cleanup time and lowered chemical exposure. Employees noticed cleaner air, and production losses dipped. Moving forward, companies can rethink water use, adopt closed-loop systems, and ensure older chemicals are phased out in favor of safer options. Chemists shouldn’t have to choose between performance and safety — using well-studied intermediates like M-(4,5-Dihydro-3-Methyl-5-Oxo-1H-Pyrazol-1-Yl)Benzenesulphonic Acid narrows that gap.
Training goes beyond a quick class or a stack of manuals. Frontline workers and managers alike need hands-on experience handling intermediates, not just in fume hoods but through projects that connect chemical choices to outcomes. Open records, regular health monitoring, and independent oversight remind everyone: there’s more at stake than color on a shirt. Proper use matters, and picking reliable chemical intermediates supports not just industry facts and figures, but real people downstream.
Every product, from snacks to solvents, reacts differently to changes in its environment. Heat from a sunny storeroom, even just for a day, can spell trouble. Moisture sneaks in undetected and changes the game. Leaving a product too close to cleaning supplies isn’t just unpleasant—it poses a real safety risk. Years working with warehouse teams taught me that storage decisions make or break product quality and safety. Small oversights create big headaches later.
The best place for many products is a cool, dry spot with steady airflow. Sensitive items, especially those marked with temperature ranges, need more support. Some break down if they sit anywhere above 25°C for long. Light-sensitive materials start to lose their punch after a few days beneath harsh fluorescent bulbs. In our lab, we used black-out curtains for samples that would break down otherwise.
Some pharmaceuticals need refrigeration from the second they leave the manufacturer to the moment they reach the customer. That means climate monitoring every step of the way. Skipping a temperature log or opening the fridge even briefly can mean the loss of an entire shipment. According to the World Health Organization, around 50% of vaccines lose potency worldwide each year because of improper temperature control. That’s a heart-sinking statistic.
Moisture levels matter too. Cardboard boxes warp and lose strength in moist storage rooms. Powders clump together and become useless. We learned this first-hand while shipping calcium supplements—they arrived fused into concrete-like blocks instead of free-flowing powder, just from a week of damp storage before shipping.
Cross-contamination caused by storing chemicals and foods together happens more than most expect. The pungent scent of one product can seep unmistakably into another, even through sealed packaging. Food safety experts flag this as a common source of recall.
Handling starts with knowledge. Staff who know what’s inside a drum or box act faster when something spills or leaks. Training isn’t just about checking boxes; it’s about culture. In our facility, sharing stories of what went right and wrong with real-life handling made a bigger difference than reading from a manual. Proper labels, gloves, and eye protection prevent most injuries and mix-ups.
Documentation supports trust across the supply chain. A clear chain of custody, batch numbers, temperature logs, and signed-off delivery slips mean problems can be traced and fixed fast. In the food industry, the Food Safety Modernization Act set stricter documentation requirements after a string of bacterial outbreaks traced back to gaps in record-keeping. Many suppliers now use QR codes for instant traceability, which helps solve problems within hours instead of days.
Remote sensors now send real-time alerts when temperatures shift. Automated inventory tracking catches expired products before they leave the shelf. Tougher packaging stands up to warehouses that don’t have perfect climate control. But no gadget replaces regular training, honest communication, and steady vigilance. Small improvements—like checking storage room thermostats, rotating stock, or setting clear “do not stack” warnings—have a measurable impact.
Storage and handling might seem like routine work, but it holds the key to product integrity and safety. Paying attention here translates to trust down the line, fewer recalls, and better outcomes for everyone.
Every workplace I’ve set foot in, from warehouses to lab classrooms, carries at least one chemical that could mess up your health if not respected. It's easy to get used to the idea that a bottle with a label means someone already thought about what’s inside and it can’t be that bad. I learned otherwise in my late teens, working as a janitor scrubbing classroom floors: my hands stung for days after handling an unlabeled bottle. Even common cleaning chemicals can trigger rashes or headaches. If a label lists hard-to-pronounce words or hazard symbols, it ups the stakes. People need to take chemicals seriously, not just for their own well-being, but for the folks around them too.
Labels throw out warnings like “corrosive,” “toxic,” or “carcinogen.” Regulatory agencies require this for a reason. It’s not about legal coverage, it’s about keeping workers out of ER waiting rooms. If you spot a skull-and-crossbones or flame symbol, don’t play guessing games. Learn what those signs really mean on the SDS—Safety Data Sheet. These documents seem thick, but the details on safe handling and emergency actions are gold. The experience of cleaning up a spilled solvent, not knowing if the fumes were toxic, taught me that preparation beats regret any day.
Bleach and ammonia sit under kitchen sinks everywhere, but if mixed, turn into a lung-scorching cloud of gas. The danger lies in normalizing something just because everyone uses it. Most serious accidents start with the phrase, “I’ve done this a hundred times and nothing happened.” I saw a friend ignore instructions mixing two cleaning agents, only to start coughing and turn pale. It took a rushed trip outdoors to clear his lungs. Lesson learned: ask questions, check the material safety info, never rely on routine to keep you safe.
Company handbooks usually spell out gloves, goggles, masks, even aprons. Over the years, I’ve seen these rules get ignored—when supervisors aren’t looking, folks ditch the “hot” gloves because they’re awkward. That hour saved could cost a week recovering from a chemical burn or respiratory issue. Nitrile gloves, face shields, and solid ventilation aren’t there to make your day slower, they save skin and lungs. My hands came out in blisters after one job swapping to regular latex gloves. After that, I never skimped on real gear again, no matter how clumsy it felt.
Most chemical problems don’t happen in isolation. Sloppy labeling, crowded storage shelves, or poor ventilation turns a minor hazard into something major. During college, a poorly ventilated storeroom once filled our entire hallway with toxic fumes—because the vent was blocked and containers were stacked to the ceiling. Good ventilation, clear signs, routine equipment checks, and regular safety drills make for a safer space. Newcomers need real training, not just checklists. Everyone should feel safe flagging an unsafe practice, and leadership needs to listen.
Safety comes from personal responsibility backed by real resources. Companies must keep SDSs on hand, maintain the right gear, inspect regularly, and train workers to spot hazards early. Employees must read up, ask questions, dress right, and avoid shortcuts. The law supports safe work environments, but habits and awareness fill in the gaps. My experience reminds me: no job moves so quickly that it’s worth risking your health over.
Anyone who has worked in a lab or with industrial materials knows the headache that comes from a substance that just won’t dissolve as expected. Early in my career, I spent weeks trying to run a straightforward formulation, only to have the active ingredient clump in the vessel, refusing to go into solution. It taught me right away that the solubility profile isn’t just an academic topic — it determines how useful a compound turns out to be in the real world.
Solubility depends on both the nature of the molecule and the properties of the solvent. For instance, a salt will blend quickly in water but barely budge in oil. In contrast, something more lipophilic just slides into non-polar solvents and barely registers in water. Many pharmaceuticals rely on this distinction: some go through the body fast because water sweeps them along, while others linger in tissues. Too little solubility in water blocks bioavailability, choking off absorption and making a new drug nearly worthless.
Some manufacturers hope broad claims about compatibility will sell a product. Experience says it’s always better to ask for solubility data and check for yourself. Compatibility profiles cover more than just “mixes well;” they include chemical reactivity, breakdown products, and long-term storage stability. For example, someone may dissolve this compound with a certain surfactant, only to discover later that the combination breaks down into unwelcome side products once heat comes into the picture. Proper testing includes shaking, heating, stirring, and freezing samples alongside the usual dissolution trials.
A few years ago, one colleague tried to shortcut this process, mixing a compound straight into a solvent blend recommended on a data sheet. Steady precipitation followed within hours, followed by complaints from quality control. Lesson learned: chemical reality matters more than marketing or shortcuts. Always confirm, especially with compounds known for forming hydrates or showing temperature-dependent solubility curves.
Sometimes, enhancing solubility means tweaking the environment instead of the compound itself. I’ve found that simple steps, like changing the salt form or adjusting pH, create a big difference. For example, basic drugs dissolve better at low pH, while acidic ones go further at higher pH. Cosolvents help too, especially when dealing with stubbornly hydrophobic molecules. Ethanol or glycerol often save the day when water alone falls short.
Compatibility checks don’t stop at mixing—storage conditions, processing temperatures, and even exposure to light all affect success. Plenty of pharmaceutical recalls link right back to skipped compatibility testing, often costing millions and damaging reputations. I’ve worked on cross-functional teams where the chemists, engineers, and safety specialists all get a say before green-lighting a formulation. It isn’t just about ticking boxes. Each perspective uncovers new pitfalls, from unexpected crystallization to slow polymerization that only reveals itself months later.
People depend on safe products that work every time, whether it’s a medicine, food additive, or industrial catalyst. Informed solubility and compatibility data guide smarter choices, preventing failures that disrupt production lines or harm patients. Careful experimentation, supported by published research and shared experience, paves the way for solutions that perform as promised. In the end, the time spent running a few more tests beats the cost of cleaning up a crisis.
Anyone buying food supplements, chemicals, CBD, or skincare has probably heard the question, “Do you provide a certificate of analysis for this product?” For some shoppers, the answer means everything. Years buying vitamins online taught me to never trust a label without proof. Sometimes the label promises pure ingredients, but nobody wants to gamble with their health or waste money on fillers.
A certificate of analysis—or COA—shows exactly what’s in the bottle or bag. Labs test products and print their findings: ingredient percentages, purity levels, contamination checks, and more. It’s not marketing fluff. It’s real, measurable, and tied to that specific batch. If heavy metals, pesticides, or fake additives show up, the report calls it out. Many buyers never notice how easy it is for things to sneak in, from lead dust in imported herbs to cheap bulking agents in powders. Brands that skip lab reports cut corners. Those who share results have nothing to hide.
Purity isn’t just a buzzword for athletes and health nuts. The 2008 melamine milk scandal in China killed children. Years later, contaminated dietary supplements in the U.S. forced mass recalls. These stories aren’t ancient history. Something as small as a vitamin C tablet can contain traces of toxic metals. That’s not something shoppers can spot with the naked eye, but a COA exposes anything amiss. Renowned companies like NOW Foods and Pure Encapsulations publish third-party test results to build trust—because trust pays off in the long run. According to the U.S. Food and Drug Administration, supplement recalls due to impurities hit new highs in the past decade, showing the risks of skipping independent testing.
The companies actually providing a COA signal they value customer health and care about their name. They invite consumers to look at lab test sheets. People like me want to know the lot number matches, and that what’s inside really lines up with claims on the front label. If a business dodges simple requests for lab reports, customers have every right to walk away. Each certificate should show the lab name, signatures, dates, and a full breakdown of findings. Credible vendors post these results online or give them on request, and they welcome questions about contaminants, allergens, and potency. It’s basic honesty most expect, especially if a product goes in the body or on the skin.
Shoppers and businesses can push for more transparency from day one. Ask for lab reports before buying, and share information with your friends if something looks odd. Learn to read the key numbers—like ppm (parts per million) of heavy metals, or the percentage of active compounds. People with allergies should check for trace ingredients. Manufacturers should partner with accredited, independent labs—not just the cheapest bidder in town. The demand for honest testing affects trends in the entire industry. Over time, more buyers will expect and insist on seeing real lab work, driving change at every level. That’s how healthier choices become mainstream, and shady shortcuts start drying up.
People have every reason to keep pressing for real answers about what goes into their supplements, raw materials, or skincare products. A COA isn’t a guarantee of perfection, but it’s an honest snapshot, and right now, it’s the best tool for weeding out empty promises and dangerous shortcuts. The smartest brands know that handing over the facts does more for their reputation than any glossy advert ever could.
| Names | |
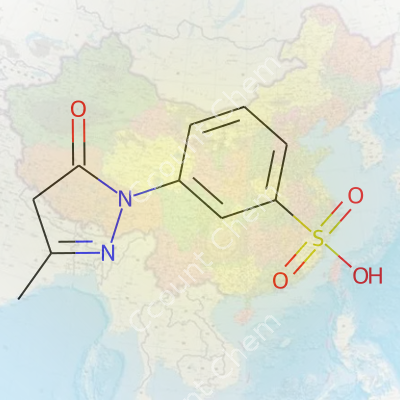
| Preferred IUPAC name | 4-[4,5-Dihydro-3-methyl-5-oxo-1H-pyrazol-1-yl]benzenesulfonic acid |
| Other names |
M-(4,5-Dihydro-3-Methyl-5-Oxo-1H-Pyrazol-1-Yl)Benzene Sulphonic Acid 3-(4,5-Dihydro-3-Methyl-5-Oxo-1H-Pyrazol-1-Yl)Benzenesulfonic Acid m-(4,5-Dihydro-3-Methyl-5-Oxo-1H-Pyrazol-1-yl)Benzene Sulphonic Acid |
| Pronunciation | /ɛm-(ˈfɔːr,ˈfaɪv-daɪˌhaɪdrəʊ-ˈθriː-ˈmiːθɪl-ˈfaɪv-ˈəʊksəʊ-wʌn-eɪtʃ-paɪˈræzɒl-wʌn-aɪl)-ˈbɛnziːnsʌlˌfɒnɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 24170-66-7 |
| 3D model (JSmol) | `4Z5OWz4tw7a7A0n6B |
| Beilstein Reference | 1817547 |
| ChEBI | CHEBI:72898 |
| ChEMBL | CHEMBL382542 |
| ChemSpider | 20894022 |
| DrugBank | DB08797 |
| ECHA InfoCard | 13-2319309336-51-0000 |
| EC Number | EC 405-040-6 |
| Gmelin Reference | 87786 |
| KEGG | C18538 |
| MeSH | D017346 |
| PubChem CID | 22275494 |
| RTECS number | DJ1226000 |
| UNII | J6U3991E81 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID1063574 |
| Properties | |
| Chemical formula | C10H10N2O4S |
| Molar mass | 260.27 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Odorless |
| Density | 1.42 g/cm³ |
| Solubility in water | soluble |
| log P | 0.01 |
| Acidity (pKa) | 2.0 |
| Basicity (pKb) | 5.94 |
| Refractive index (nD) | 1.607 |
| Viscosity | Viscous liquid |
| Dipole moment | 5.1 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 417.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1502.6 kJ/mol |
| Pharmacology | |
| ATC code | A16AX07 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313 |
| Flash point | Flash point: >110°C |
| NIOSH | NIOSH: QJ4900000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 400 mg |
| Related compounds | |
| Related compounds |
4,5-Dihydro-3-methyl-5-oxo-1H-pyrazole Benzenesulfonic acid 3-Methyl-1H-pyrazol-5(4H)-one 1-(4-Sulfophenyl)-3-methyl-4,5-dihydro-1H-pyrazol-5-one M-(3-Methyl-5-oxo-1H-pyrazol-1-yl)benzenesulphonic acid |