Chemists started exploring sulfonic acids in the early 19th century, chasing the promise of enhancing solubility and reactivity for industrial and research tasks. Hexanesulfonic acid’s roots trace back to the steady, sometimes bumpy, march of synthetic organic chemistry that cranked out new reagents for chromatography, pharmaceuticals, and industrial additives. Back in the 1960s, the emergence of this compound changed the game for reversed-phase ion-pair chromatography. Researchers needed a better way to separate tricky molecules, and C6-sulfonic acids filled the niche with their reliable, consistent interaction profiles. Scientific literature from the late 20th century shows how quickly labs adopted hexanesulfonic acid, moving it from chemical catalog obscurity to a regular item on requisition lists at both academic and industrial labs. That’s no small leap for any specialty reagent.
Most people working in analytical chemistry have encountered hexanesulfonic acid under a few trade and research names, though the structure never changes—a six-carbon backbone, terminating in a sulfonic acid head. Whether you pick up a bottle marked as 1-hexanesulfonic acid or as hexane-1-sulfonic acid, it means you’re dealing with a solid, white, crystalline chemical, chosen for its strong acidity and remarkable solubility in water and polar solvents. Most commercial labs buy it for use as a mobile phase additive, most notably in high-performance liquid chromatography (HPLC). The demand keeps climbing in applications where pH stability and ionic strength matter more than purity for the sake of purity. Reliable suppliers keep this product available both in research-grade and analytical-grade formats, ensuring tight controls on contamination and byproduct levels.
Hexanesulfonic acid can fool a chemist at first glance. It looks like many common organics, but don’t confuse its neutral appearance for mildness—it brings a robust acidity, with a pKa dipping well below zero, firmly in the realm of strong acids. The compound’s molecular formula, C6H14O3S, lands it at a molecular weight just below 182 g/mol, and it shows up as a free-flowing crystalline powder. Solubility anchors its appeal; it dissolves without complaint in water, ethanol, and methanol. The melting point hovers in the range of 150-160°C, usually without decomposition at standard storage conditions. Its sulfonic acid group resists air oxidation, a feature that matters when users need consistent performance without worrying about in-bottle degradation.
Anyone buying from a reputable chemical supplier will see detailed labeling and specifications—often purity above 99%, low residual solvent levels, and definite pH ranges for solutions. Each lot ships with safety data, batch analytics for trace impurities, and recommended shelf-life conditions, stamped right onto the datasheets. People who work in ISO-certified environments depend on these certifications for traceability. Certificates of analysis list everything from heavy metal content to residual water levels and organic impurities, reflecting a market-wide trend toward complete chemical transparency. Transparent traceability is important for any lab working toward regulatory approvals or quality certification.
The process for producing hexanesulfonic acid typically begins with n-hexane. Industrial chemists introduce sulfur trioxide or fuming sulfuric acid to n-hexane, setting off a sulfonation reaction that attaches the sulfonic acid group to the end of the carbon chain. The operators must keep conditions controlled—overheating or prolonged reaction times can produce unwanted polysulfonation or break down the backbone. Follow-up stages wash and purify the crude product, sometimes using aqueous workups, then crystallization from polar solvents like water or methanol. The result is an off-white or colorless solid that undergoes analytical verification for purity and structure before packaging. The whole route demonstrates how much modern specialty chemistry depends on careful control and efficient downstream purification.
Hexanesulfonic acid earns its keep through versatility. Many organic syntheses harness this compound for swapping out counterions or forming temporary salts. Its sulfonic group can undergo neutralization to yield the sodium or potassium salt, both highly valued in analytical labs for their stability in aqueous solutions. Specialized modifications, such as converting the acid to an ester, see use in developmental work with surfactants or pharmaceutical intermediates. In analytical chemistry, using the sodium salt in ion-pair chromatography allows separation of basic drugs and peptides that would otherwise streak or tail on reversed-phase HPLC columns. Its chemical stability means it can survive tough processing, only rarely requiring protection during complex, multistep syntheses.
Walk into any industrial catalog or scientific database, and you’ll spot hexanesulfonic acid listed under aliases like 1-hexanesulfonic acid, n-hexane-1-sulfonic acid, or just C6 sulfonic acid. Some legacy literature might call it caproic sulfonic acid, though that runs the risk of confusion with caproic acid, which carries a carboxylic acid rather than a sulfonic group. Among HPLC suppliers, look for sodium hexanesulfonate or potassium hexanesulfonate—these names pop up often in mobile phase preparations. Names change but the purpose hardly shifts: providing a reliable, strong-acid additive for chromatographic and synthetic work.
People underestimate strong acids just because they aren’t fuming or smoking. Hexanesulfonic acid packs a punch if mishandled. It requires gloves, goggles, and standard laboratory ventilation. Spill protocols look a lot like those for sulfuric acid—neutralization with sodium bicarbonate, careful washing, and no shortcuts on personal protection. Container labeling always highlights its corrosivity to eyes, skin, and mucous membranes. Researchers and operators follow the hazard codes, referencing Safety Data Sheets every time a new shipment arrives, and waste streams stay separated from organics and bases. Storage recommendations call for cool, dry shelving with no access to incompatible materials like strong oxidizers or reducing agents. The same chemical stability that helps in chromatography also means accidental spills stick around until they’re consciously cleaned up.
Most chemists discover hexanesulfonic acid through its starring role in reversed-phase ion-pair chromatography. The sulfonate anion teams up with positively charged drug molecules, increasing their retention and sharpness on silica-based columns. Pharmaceutical labs use it for separating basic compounds and peptides, where many other additives fail to deliver either clarity or reproducibility. Proteomics researchers use the sodium salt variant for stabilizing certain peptides and proteins, helping to pull apart mixtures that otherwise smear into undifferentiated messes. Other industries, including wastewater analysis and specialty surfactant synthesis, take advantage of its high acidity and water solubility for analytical and synthetic purposes. Over time, its job description has stretched out, showing up in quality control labs, environmental monitoring projects, and even forensic testing kits.
Research labs push the boundaries of hexanesulfonic acid every year. Custom formulations for supercritical fluid chromatography, new derivative salts for better stability in biocompatible applications, and greener production protocols all reflect growing interest from both academia and industry. Pharmaceutical R&D teams test analogs with slightly longer or shorter carbon chains, hunting for additives that give better peak resolution or shorter run times. Analytical scientists develop novel detection methods, using the compound’s strong UV absorbance in indirect detection schemes. Instrument manufacturers work closely with chemical suppliers, setting technical benchmarks for both purity and reproducibility. As chromatography trends toward higher-throughput and more complex sample matrices, expectations for this workhorse additive only rise.
Though structurally robust, hexanesulfonic acid brings some safety baggage. Published research highlights the concern about local tissue damage upon direct contact, much like other sulfonic acids of similar chain lengths. Oral and dermal exposure in animal models shows that the main risk ties back to general strong acid corrosivity rather than unique or unexpected metabolic byproducts. Industrial hygiene guidelines keep exposure limits conservative until long-term data can fill in the blanks. In regulated environments, especially pharmaceutical manufacturing, operators monitor airborne dust and enforce spill cleanup protocols to avoid accidental skin or respiratory contact. Research into chronic toxicity and biodegradability continues, tracking not only acute health risks but also potential impacts on soil and water quality downstream. Environmental safety officers pair those data with robust accident and containment training to keep risks managed.
Looking forward, hexanesulfonic acid stands to influence how scientists and process engineers address separation challenges in high-throughput and environmentally sensitive workflows. HPLC continues to expand its reach into food safety, clinical diagnostics, and environmental tracing, while regulatory agencies keep pushing for tighter detection of low-level contaminants and metabolites. The demand for additives that deliver both reliable performance and lower environmental impact keeps growing. Chemists explore new synthetic routes that reduce byproduct formation and energy use, working side by side with process engineers to scale up production without stepping up the ecological burden. Advances in recycling and purification likely carve out new spaces for this reagent where single-use chemicals once ruled. The compound’s ability to function under tough analytical and operational conditions means its shelf life in the research and production world will last for many years, shaped by the twin demands of smarter chemistry and careful safety stewardship.
Most people rarely think about hexanesulfonic acid, yet this chemical influences many environments, from research labs to pharmaceutical plants. As a writer who’s spent years diving into chemical supply chains and product development, I’ve seen firsthand how even a simple-looking molecule can unlock advances across whole industries. Hexanesulfonic acid stands out because of its sulfonic functional group and a straightforward six-carbon chain; this structure opens doors in analytical chemistry, manufacturing, and more.
Labs depend on separation techniques to pick apart complex mixtures. Hexanesulfonic acid acts as an effective ion-pairing reagent in both high-performance liquid chromatography (HPLC) and reversed-phase liquid chromatography. Scientists use it to sharpen the resolution between molecules that resist traditional separation because of their charges or similar structures. My experience working alongside analytical chemists made clear how choosing the right ion-pairing agent often marks the difference between clear results and a blurry mess. Sulfonic acids like this one help isolate amino acids, pharmaceuticals, nucleotides, and peptides. The ease with which it forms pairs with positively charged analytes gives it a clear edge in bioanalysis.
Modern medicine relies on exceeding strict standards for identity and purity. Pharmaceutical manufacturers use hexanesulfonic acid when screening drugs and verifying regulatory compliance. Think of quality assurance teams running countless samples—drug precursors, synthesized proteins, even blood plasma extracts. Hexanesulfonic acid helps reveal trace-level impurities at low levels, preventing recalls and keeping patients safer. Reliable quality controls form the backbone of trust in medicine, and I’ve witnessed how disruptions in reagent supply can slow down entire labs.
Flexibility often makes the difference in experimental design. Some researchers pick hexanesulfonic acid for just the right balance between hydrophobicity and charge, letting them tweak their chromatography setups for novel compounds. Choosing the correct chain length in sulfonic acids can make or break a separation protocol, and researchers sometimes substitute similar reagents to match their target molecules—yet hexanesulfonic acid keeps showing up on lab benches thanks to its reliability. Technique-intensive fields like proteomics benefit because difficult peptides can finally be separated efficiently.
Clean water matters in every community, and environmental labs rely on robust chemical tools. Hexanesulfonic acid finds its way into testing soil and water for contaminants, such as pesticides and herbicides. Simple, reproducible sample prep can reduce environmental health risks and alert communities sooner to potential threats. As pollution testing grows in importance, the need for steadfast, trustworthy reagents like this one follows.
Products made with or analyzed by hexanesulfonic acid draw scrutiny from buyers demanding high transparency. Anybody handling the chemical needs clear safety guidelines and quality certifications. Consistent, high-purity batches lower the risk of analytical error or dangerous exposure, particularly in large-scale industrial or food safety settings.
Rising demand for precise analysis and safe pharmaceuticals keeps hexanesulfonic acid in focus. Responsible makers seek environmentally friendlier production routes. Chemists favor suppliers who can prove both purity and traceability, making regulation and documentation an ongoing priority in the supply chain. Increased education for lab professionals on safe handling could curb accidental spills or misuse, which strengthens trust in the final product—be it clean water, safe food, or life-saving medicines.
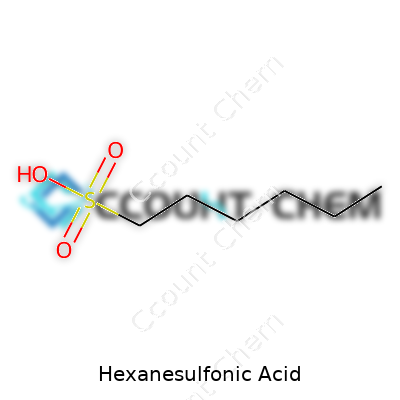
Hexanesulfonic acid isn’t just a tongue twister for chemists; it’s a solid staple in labs and industry alike. This compound contains a six-carbon straight chain attached to a sulfonic acid group. The structure is simple but powerful: a hexyl group bonded to a sulfonic acid functional group. Chemically, it goes by the formula C6H13SO3H. That means six carbons, thirteen hydrogens, sulfur, and three oxygens capped off with an acidic hydrogen. It sounds dry, but this formula defines how the molecule interacts in real-world applications.
In my work supporting chromatography, I’ve run into this acid often. Labs lean on hexanesulfonic acid as an ion-pairing agent during liquid chromatography. The chain length gives it extra reach, letting it tie up with organic molecules and improve how things separate in a column. If the formula strays—adding or dropping a carbon, for example—the interaction changes, and a method that normally runs smooth can hit bumps. Specific molecular makeup like C6H13SO3H delivers repeatable, predictable results.
Chemists value reliability, so a few real-world facts come into play. Hexanesulfonic acid as C6H13SO3H is stable when handled properly. The sulfonic acid group confers strong acidity, giving it teeth for chemical reactions. Mishandling can trigger skin and eye irritation. I always suit up with gloves and goggles. Chemical supply sheets list its corrosive nature—it loves water but can damage metals and tissues if ignored.
Accidental contact with the concentrated acid means rinsing immediately with water and seeking help. Modern labs take these facts seriously because a single formula error during handling is enough to put safety at risk. The chemical's stability in storage—glass bottles, dry shelves, cool air—keeps it ready for analysis any day it’s needed.
Many overlook the critical role of knowing what’s inside the container. Guessing or mixing up similar compounds—say, swapping hexanesulfonic acid for octanesulfonic acid—creates headaches ranging from poor instrument performance to wasted materials, and even accidents. The molecular structure spelled out in C6H13SO3H tells scientists how the model will behave, what precautions to follow, and how to dispose of waste.
Quality control relies on careful verification. In regulated environments such as pharmaceuticals or water analysis, substituting the wrong chain length could impact patient health or environmental reporting. Small mistakes introduce big risks. A lab that masters these basics earns trust—and keeps surprises from cropping up in critical applications.
Access to verified chemical stocks remains essential. Online sources sometimes cut corners, so I check for detailed certificates of analysis before purchase. Training newcomers on these details pays back tenfold, reducing wasted runs and exposure incidents.
Manufacturers and suppliers have a part to play, offering easy-to-read labeling and safety instructions. Fact-based training, clear labeling, and careful handling protocols keep both results consistent and workers safe from harm. Public databases and professional societies offer updated fact sheets and best practice guides, supporting those in industry or academia who want to double-check formulas.
Hexanesulfonic acid doesn’t turn up in daily life outside a chemistry lab. The compound, known for its utility in chromatography and certain chemical syntheses, often comes as a clear or pale liquid. Its strong sulfonic group means researchers tap it for separating chemicals, not whipping up consumer products. Despite its regular use in science settings, concerns about its safety still loom.
Touching or inhaling hexanesulfonic acid poses some proven risks. Lab safety data sheets don’t mince words: this substance earns a spot among irritants. Skin and eyes don’t handle splashes well, sometimes reacting with redness, burns, and lasting discomfort if not rinsed off quickly. Breathing in the vapors can set off coughing and shortness of breath. Ingesting the stuff—accidental or not—could unleash burns and stomach pain.
Hazard ratings classify hexanesulfonic acid as corrosive. That label sticks because it chews through living tissue. A simple glove puncture or a fumbled bottle can mean more than inconvenience. During university laboratory classes, I saw a student spill something similar. She underestimated the sting from just a few droplets—and the nurse’s flush-station routine left no one eager to take shortcuts again.
Toxicity, in the world of chemistry, splits into acute and chronic categories. For acute exposure, hexanesulfonic acid’s effects fall right in line with strong acids; quick contact with skin or eyes means pain and tissue damage. No one would recommend testing its limits on purpose. For chronic exposure, the story isn’t as well-documented as with famous toxins like mercury or lead. That doesn’t give it a free pass. Data shows compounds similar to hexanesulfonic acid can harm lungs or digestive tracts after repeated low-level encounters.
Animal toxicity studies on hexanesulfonic acid look sparse. Even with the gaps, the acid’s harsh nature kicks safety habits into gear. Lab teams stick to fume hoods, goggles, and gloves—they’ve seen the results of neglect. None of this matches the toxicity of cyanide or arsenic, but hexanesulfonic acid’s risks demand respect.
Chemicals don’t stay in the lab forever. Sometimes, improper disposal lets acids like hexanesulfonic acid trickle into drains or water systems. Sulfonic acids often resist breaking down, raising alarms about their effect on aquatic life or soil quality. Fish and water-dwellers tend to feel harsh acids more than humans, with fatal results at only modest concentrations. Regulations expect careful handling for disposal, and those rules develop because past mistakes left dead rivers or unhealthy groundwater.
Training and good habits stop most accidents before they start. Putting on gloves isn’t just for looks, and storing the acid away from anything reactive goes beyond rule-following. Emergency showers and flush stations earn their price every time someone misjudges their pour. Strong labeling and regular safety drills in workplaces highlight how to act fast in emergencies.
Researchers look for alternatives with milder profiles, and manufacturing facilities keep reviewing best practices. Waste collection services increasingly demand full documentation to prevent underground leaks. In my own experience, the labs that never cut corners are the ones with the fewest stories about ER visits or ruined samples. That example offers a lesson worth repeating outside the lab.
Hexanesulfonic acid isn’t like table salt or sugar. Its chemical properties put it in a different league. With its strong acidic character and readiness to react, a careless approach invites trouble. My background in chemical supply taught me one thing above all: safe storage forms the backbone of any lab’s safety culture. You might find a bottle on a dusty shelf in a forgotten storeroom, but that doesn’t mean it belongs there.
The point isn’t only about product purity or performance. This compound can trigger reactions with everyday materials. Corrosion eats through shelves, jars, and even your safety budget. So, step one is paying attention to where and how you put it away.
Proper storage keeps both people and products safe. The acid’s tendency to degrade or interact with moisture means dry, cool rooms work best. I’ve seen labs push drums into boiler rooms or stuff bottles near water lines to “save space.” All it takes is one leak, and you’re dealing with fumes or metal corrosion.
Ventilation matters. Fumes build up even in sealed containers—especially if caps come loose or materials break down. Walk into a cramped chemical closet without airflow, and you’ll understand why stale air and chemical vapors shouldn’t mix. Keep it away from sunlight, too. UV can set off unwanted reactions.
Not every container works with strong acids. Early in my career, a shipment arrived in recycled plastic jugs. The acid eventually ate through the plastic, and we lost both product and peace of mind. Glass or specially lined plastic containers handle the challenge much better. Look for secure, chemical-resistant lids—no jury-rigged solutions.
Labels need to stay clear and readable. More than once, I’ve seen faded labels lead to guessing games, which only invite accidents. Permanent, waterproof markers and printed warnings let everyone know what’s inside at a glance.
Mixing hexanesulfonic acid with substances on the “do not touch” list risks fire or toxic gas. Simple enough to say, hard to use as a rule unless you regularly check what sits nearby. I’ve found it pays off to slot acids away from anything alkaline, oxidizing, or flammable.
Unexpected spills teach hard lessons. Once, a leaking jar meant cleaning up with proper gloves, goggles, and a spill kit. Standard household cleaners won’t cut it—neutralizing acids takes specific substances like sodium bicarbonate. Emergency eyewash and showers near storage locations add another layer of safety. It’s not enough to stash them “somewhere nearby”; keep the area free of clutter so you can reach help without stumbling.
Containers and their labels don’t last forever. Scheduled inspections discover cracks, leaks, or slow corrosion. I’ve walked past storage lockers with pooling residue long before anyone noticed serious damage. A quick monthly scan can head off trouble for both the crew and your chemical stash.
Anyone with access should know the drill. Watching an experienced colleague handle these materials stays with you longer than reading policies. Discuss safe handling in every meeting, refresh knowledge on material safety data sheets, and walk new team members through the right steps in person. Culture, not just checklists, keeps accidents at bay.
Hexanesulfonic acid isn’t as familiar as acetone or bleach, but its role in lab procedures and analytical chemistry lands it on many workbenches. The trouble starts with its seriousness as an irritant—skin, eyes, and lungs usually pay the price for carelessness. I’ve seen what can happen when safety slips: tight chests, red skin, and faces hunting for eyewash stations. Once exposure happens, nobody wants a repeat.
Looking at personal gear, the basics still matter most. Nitrile gloves keep acid contact off your skin, and chemical splash goggles keep undergraduates from sprinting to sinks with stinging eyes. Lab coats block splashes that hit your arms or chest. Closed shoes keep feet safe. I’ve kept an acid mark on my ring finger for months after skipping gloves—lesson learned and not forgotten.
Hexanesulfonic acid gives off fumes that sting and hang in the air. I’ve watched foggy goggles slow people down after an hour in a poorly ventilated room. Fume hoods keep those vapors out of your breathing space. Always handle this reagent inside a hood, not just on a bench by an open window. With so much talk on lab budgets, good ventilation can slip through the cracks. Yet fixing it always costs less than a trip to urgent care.
Setting this acid on any shelf will invite trouble. It belongs in bottles with visible, sturdy labels. Corrosive cabinet storage, away from strong bases and oxidizers, stops the kind of chemical accidents nobody wants to see. Sturdy secondary containment trays help trap leaks. I’ve seen cabinets where acids sat next to bases just because the team wanted tidy rows—cleanup after a spill taught them otherwise.
Working with hexanesulfonic acid goes beyond mixing and measuring. Spills, even small ones, require immediate attention. Absorbent pads and neutralizing agents—sometimes simple sodium bicarbonate—sit nearby for a reason. Trained eyes catch spills faster, and everyone in the lab needs to know where the cleanup kit hides. I remember a time when confusion and slow responses made a minor drop spread into a sticky mess.
Dumping hexanesulfonic acid down the drain sounds quick, but it risks both pipes and water supplies. Instead, corrosive waste containers specifically marked for acids keep disposal safe and separate. Regular pickups by certified hazardous waste handlers prevent buildup and confusion. One missed waste pickup in my lab set off alarms and shut down work until the barrels moved out.
Every researcher or technician benefits from regular walk-throughs and safety reminders. Reading material safety data sheets offers knowledge beyond rumors swirling around the lab. Refresher courses and honest conversations after close calls build a stronger safety culture than any poster on the wall.
The steps to keep safe around hexanesulfonic acid tie back to old-school wisdom: stay alert, respect what’s in your hands, and don’t cut corners. Making safety a daily habit—not just a checklist—keeps accidents rare and everyone headed home in one piece.
| Names | |
| Preferred IUPAC name | hexane-1-sulfonic acid |
| Other names |
1-Hexanesulfonic acid n-Hexanesulfonic acid Hexylsulfonic acid Hexan-1-sulfonic acid |
| Pronunciation | /ˌhɛk.seɪnˌsʌlˈfɒn.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 506-59-2 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Hexanesulfonic Acid**: ``` C(C(C(C(C(C)S(=O)(=O)O)H)H)H)H ``` |
| Beilstein Reference | 471220 |
| ChEBI | CHEBI:24567 |
| ChEMBL | CHEMBL1228962 |
| ChemSpider | 87294 |
| DrugBank | DB11107 |
| ECHA InfoCard | ECHA InfoCard: 100.004.249 |
| EC Number | 272-145-9 |
| Gmelin Reference | 78497 |
| KEGG | C18702 |
| MeSH | D006623 |
| PubChem CID | 11497 |
| RTECS number | GN7700000 |
| UNII | 3H4ZNF998M |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID8048751 |
| Properties | |
| Chemical formula | C6H14O3S |
| Molar mass | 214.32 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.04 g/mL at 25 °C |
| Solubility in water | Soluble |
| log P | -1.3 |
| Vapor pressure | 0.0083 mmHg (25°C) |
| Acidity (pKa) | 1.92 |
| Basicity (pKb) | 1.03 |
| Magnetic susceptibility (χ) | -0.000044 |
| Refractive index (nD) | 1.428 |
| Viscosity | 1.2 mPa·s (25 °C) |
| Dipole moment | 2.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 307.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -888.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -897.4 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H314 |
| Precautionary statements | P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-1 |
| Flash point | 36 °C |
| Autoignition temperature | 365°C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): 600 mg/kg (rat, oral) |
| NIOSH | MW3850000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 500 mg/L |
| Related compounds | |
| Related compounds |
Methanesulfonic acid Ethanesulfonic acid Propanesulfonic acid Butanesulfonic acid Pentanesulfonic acid Heptanesulfonic acid Octanesulfonic acid |